A nano car with molecular 4-wheel drive
Reduced to the max: the emission-free, noiseless 4-wheel drive car, jointly developed by Empa researchers and their Dutch colleagues, represents lightweight construction at its most extreme. The nano car consists of just a single molecule and travels on four electrically-driven wheels in an almost straight line over a copper surface.
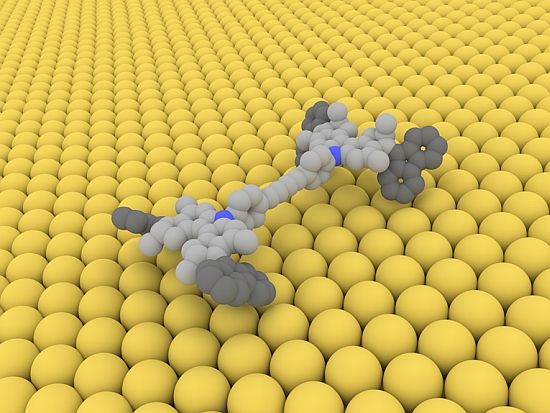
Measuring approximately 4x2 nanometres the molecular car is forging ahead on a copper surface on four electrically driven wheels.
Empa
To carry out mechanical work, one usually turns to engines, which transform chemical, thermal or electrical energy into kinetic energy in order to, say, transport goods from A to B. Nature does the same thing; in cells, so-called motor proteins – such as kinesin and the muscle protein actin – carry out this task. Usually they glide along other proteins, similar to a train on rails, and in the process “burn” ATP (adenosine triphosphate), the chemical fuel, so to speak, of the living world.
A number of chemists aim to use similar principles and concepts to design molecular transport machines, which could then carry out specific tasks on the nano scale. According to an article in the latest edition of science magazine “Nature”, scientists at the University of Groningen and at Empa have successfully taken “a decisive step on the road to artificial nano-scale transport systems”. They have synthesised a molecule from four rotating motor units, i.e. wheels, which can travel straight ahead in a controlled manner. “To do this, our car needs neither rails nor petrol; it runs on electricity. It must be the smallest electric car in the world – and it even comes with 4-wheel drive” comments Empa researcher Karl-Heinz Ernst.
Range per tank of fuel: still room for improvement
The downside: the small car, which measures approximately 4x2 nanometres – about one billion times smaller than a VW Golf – needs to be refuelled with electricity after every half revolution of the wheels – via the tip of a scanning tunnelling microscope (STM). Furthermore, due to their molecular design, the wheels can only turn in one direction. “In other words: there’s no reverse gear”, says Ernst, who is also a professor at the University of Zurich, laconically.
According to its “construction plan” the drive of the complex organic molecule functions as follows: after sublimating it onto a copper surface and positioning an STM tip over it leaving a reasonable gap, Ernst’s colleague, Manfred Parschau, applied a voltage of at least 500 mV. Now electrons should “tunnel” through the molecule, thereby triggering reversible structural changes in each of the four motor units. It begins with a cis-trans isomerisation taking place at a double bond, a kind of rearrangement – in an extremely unfavourable position in spatial terms, though, in which large side groups fight for space. As a result, the two side groups tilt to get past each other and end up back in their energetically more favourable original position – the wheel has completed a half turn. If all four wheels turn at the same time, the car should travel forwards. At least, according to theory based on the molecular structure.
To drive or not to drive – a simple question of orientation
And this is what Ernst and Parschau observed: after ten STM stimulations, the molecule had moved six nanometres forwards – in a more or less straight line. “The deviations from the predicted trajectory result from the fact that it is not at all a trivial matter to stimulate all four motor units at the same time”, explains “test driver” Ernst.
Another experiment showed that the molecule really does behave as predicted. A part of the molecule can rotate freely around the central axis, a C-C single bond – the chassis of the car, so to speak. It can therefore “land” on the copper surface in two different orientations: in the right one, in which all four wheels turn in the same direction, and in the wrong one, in which the rear axle wheels turn forwards but the front ones turn backwards – upon excitation the car remains at a standstill. Ernst und Parschau were able to observe this, too, with the STM.
Therefore, the researchers have achieved their first objective, a “proof of concept”, i.e. they have been able to demonstrate that individual molecules can absorb external electrical energy and transform it into targeted motion. The next step envisioned by Ernst and his colleagues is to develop molecules that can be driven by light, perhaps in the form of UV lasers.