New economic water-splitting catalyst
UNIST scientists have developed an exiting new catalyst that can split water into hydrogen almost as good as platinum, but less costly and found frequently on Earth.
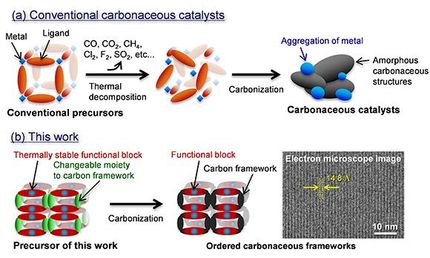
A schematic diagram illustrating the preparation of Ru@C2N is shown in the figure above. (Ruthenium: shown in gold, Carbon: shown in grey , Nitrog shown in sky-blue)
UNIST
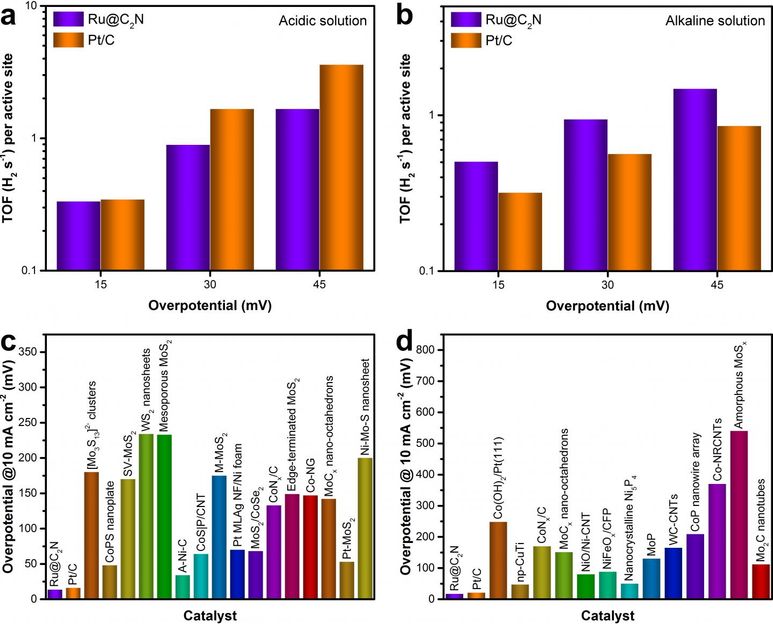
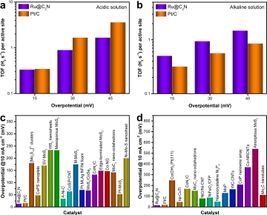
Above figure shows the comparison of the Turnover frequency (TOF) of Ru@C?N with other catalysts.
UNIST
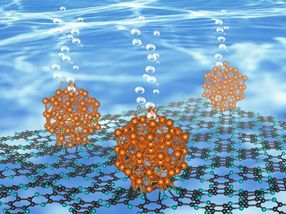
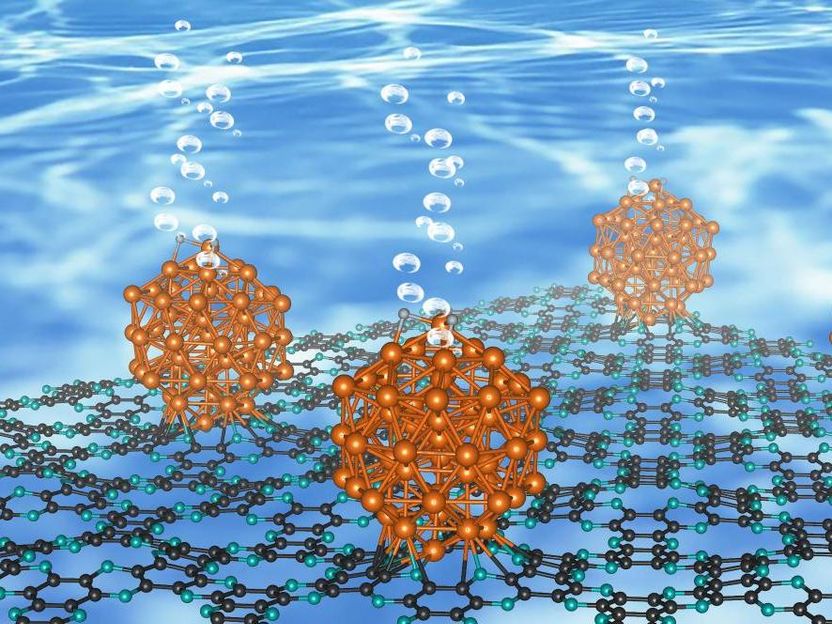
As described in the journal Nature Nanotechnology, this ruthenium-based material works almost as efficient as platinum and likely shows the highest catalytic performance without being affected by the pH of the water.
The research team, led by Professor Jong-Beom Baek of the Energy and Chemical Engineering at UNIST has synthesized Ru and C2N, a two-dimensional organic structure, to verify its performance as a water-splitting catalyst. With the aid of this new catalyst, entitled Ru@C2N it is now possible to efficiently produce hydrogen.
The technology for producing hydrogen from water requires a good catalyst for commercial competitiveness. These water-splitting catalysts must exhibit high hydrogen conversion efficiency and excellent durability, operate well under low-voltage, and should be economical.
The Pt-based catalysts used in the hydrogen generation reaction are highly expensive noble metals, resulting in additional costs and difficulty of mass production. They are also less stable in an alkaline environment.
One solution, many researchers suggest, was to build catalysts made of cheap, non-noble metals. However, because these materials corrode rapidly under acidic condition and operate at very-high voltages, productivity was limited.
The Ru@C2N, developed by Professor Baek is a high-performance material that satisfies all four commercial competitiveness of water-splitting catalysts.
This material exhibits high turnover frequency (TOF) as high as Pt and can be operated on low-voltage supply. In addition, it is not affected by the pH of the water and can be used in any environment.
The synthesis process of Ru@C2N is simple. Professor Baek and his colleagues simply mixed the ruthenium salt (RuCl2) with the monomers which forms the porous two-dimensional organic structure, C2N. The Ru@C2N catalyst is then produced after going through reduction and heat treatment processes.
The researchers used the same process to build M@C2N (M = Co, Ni, Pd, Pt) catalysts, using cobalt (Co), nickel (Ni), lead (Pb) and platinum (Pt). When comparing their efficiency of hydrogen production, the Ru@C2N catalyst exhibited the highest catalytic performance at the lowest overvoltage, as well as superior catalytic activity.
"Our study not only suggests new directions in materials science, but also presents a wide range of possibilities from basic to applied science," says Professor Baek. "This material is expected to attract attention in many areas thanks to its scientific potential."
Original publication
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.