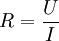To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
AmperostatAn amperostat is a control and measuring device used to keep constant the current flowing though electrolytic cells in coulometric titrations, disregarding changes in the load itself. Synonym is "galvanostat". The amperostat respond to changes in the resistance of the cell by varying its output potential: as Ohm's law shows, Product highlightthe variable system resistance and the controlled voltage are directly proportional, i.e. where
thus, an increase of the load resistance implies an increase of the voltage the amperostat applies to the load. This device differs from common constant current sources by its ability to supply and measure a wide range of currents (from picoamperes to amperes) of both polarities. See also
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Amperostat". A list of authors is available in Wikipedia. |