To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
BET theoryProduct highlight
GeneralBET theory is a well-known rule for the physical adsorption of gas molecules on a solid surface. In 1938, Stephen Brunauer, Paul Hugh Emmett, and Edward Teller published an article about the BET theory in a journal[1] for the first time; “BET” consists of the first initials of their family names. The concept of the theory is an extension of the Langmuir theory, which is a theory for monolayer molecular adsorption, to multilayer adsorption with the following hypotheses: (a) gas molecules physically adsorb on a solid in layers infinitely; (b) there is no interaction between each adsorption layer; and (c) the Langmuir theory can be applied to each layer. The resulting BET equation is expressed by (1): ![\frac{1}{v \left [ \left ( {P_0}/{P} \right ) -1 \right ]} = \frac{c-1}{v_m c} \left ( \frac{P}{P_0} \right ) + \frac{1}{v_m c} \ \ \ \ \ \ \ (1)](images/math/8/f/4/8f42583042763cd2e3c4942babb12bc6.png) P and P0 are the equilibrium and the saturation pressure of adsorbates at the temperature of adsorption, v is the adsorbed gas quantity (for example, in volume units), and vm is the monolayer adsorbed gas quantity. c is the BET constant, which is expressed by (2): 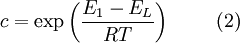 E1 is the heat of adsorption for the first layer, and EL is that for the second and higher layers and is equal to the heat of liquefaction.
Equation (1) is an adsorption isotherm and can be plotted as a straight line with 1 / v[(P0 / P) − 1] on the y-axis and φ = P / P0 on the x-axis according to experimental results. This plot is called a BET plot. The linear relationship of this equation is maintained only in the range of 0.05 < P / P0 < 0.35. The value of the slope A and the y-intercept I of the line are used to calculate the monolayer adsorbed gas quantity vm and the BET constant c. The following equations can be used: 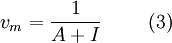 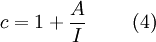 The BET method is widely used in surface science for the calculation of surface areas of solids by physical adsorption of gas molecules. A total surface area Stotal and a specific surface area S are evaluated by the following equations: 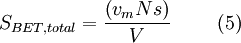 
ExamplesCement pasteBy application of the BET theory it is possible to determine the inner surface of hardened cement paste. If the quantity of adsorbed water vapour is measured at different levels of relative humidity a BET plot is obtained. From the slope A and y-intersection I on the plot it is possible to calculate vm and the BET constant c. In case of cement paste hardened in water (T=97°C), the slope of the line is A = 24.20 and the y-intersection I = 0.33; from this follows 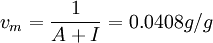 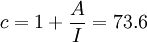 From this the specific BET surface area SBET can be calculated by use of the above mentioned equation (one water molecule covers s = 0.114nm2). It follows thus SBET = 156m2 / g which means that hardened cement paste has an inner surface of 156 square meters per g of cement. Activated CarbonFor example, activated carbon, which is a strong adsorbate and usually has an adsorption cross section s of 0.16 nm2 for nitrogen adsorption at liquid nitrogen temperature, is revealed from experimental data to have a large surface area around 3000 m² g-1. Moreover, in the field of solid catalysis, the surface area of catalysts is an important factor in catalytic activity. Porous inorganic materials such as mesoporous silica and layer clay minerals have high surface areas of several hundred m² g-1 calculated by the BET method, indicating the possibility of application for efficient catalytic materials. References
|
|||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "BET_theory". A list of authors is available in Wikipedia. |







