To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Bathochromic shiftBathochromic shift is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength (lower frequency). Product highlightThis can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism. A series of structurally related molecules in a substitution series can also show a bathochromic shift. Bathochromic shift is a phenomenon seen in molecular spectra, not atomic spectra - it is thus more common to speak of the movement of the peaks in the spectrum rather than lines.
It is typically demonstrated using a spectrophotometer, colorimeter, or spectroradiometer. Bathochromic shifts are often referred to as red shifts. Although this usage is considered informal [1], it is often used in the literature. It has no relation to Doppler shift or other wavelength-independent meanings of redshift.
See alsoHypsochromic shift, a change to shorter wavelength (higher frequency) |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Bathochromic_shift". A list of authors is available in Wikipedia. |





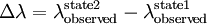 where
where