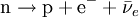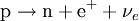To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Beta particleBeta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay. They are designated by the Greek letter beta (β). There are two forms of beta decay, β− and β+, which respectively give rise to the electron and the positron. Product highlightβ− decay (electron emission)An unstable atomic nucleus with an excess of neutrons may undergo β− decay, where a neutron is converted into a proton, an electron and an electron-type antineutrino (the antiparticle of the neutrino):
This process is mediated by the weak interaction. The neutron turns into a proton through the emission of a virtual W− boson. At the quark level, W− emission turns a down-type quark into an up-type quark, turning a neutron (one up quark and two down quarks) into a proton (two up quarks and one down quark). The virtual W− boson then decays into an electron and an antineutrino. Beta decay commonly occurs among the neutron-rich fission byproducts produced in nuclear reactors. Free neutrons also decay via this process. This is the source of the copious amount of electron antineutrinos produced by fission reactors. β+ decay (positron emission)Unstable atomic nuclei with an excess of protons may undergo β+ decay, also called inverse beta decay, where a proton is converted into a neutron, a positron and an electron-type neutrino:
Beta plus decay can only happen inside nuclei when the absolute value of the binding energy of the daughter nucleus is higher than that of the mother nucleus. Inverse beta decay is one of the steps in nuclear fusion processes that produce energy inside stars. See also
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Beta_particle". A list of authors is available in Wikipedia. |