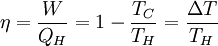To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Carnot's theorem (thermodynamics)Carnot's theorem, also called Carnot's rule is a principle which sets a limit on the maximum amount of efficiency any possible engine can obtain, which thus solely depends on the difference between the hot and cold temperature reservoirs. Carnot's theorem states:
Product highlightThe rule was an essential stepping stone towards the formulation of the second law of thermodynamics. When transforming thermal energy into mechanical energy, the thermal efficiency of a heat engine is the percentage of energy that is transformed into work. Thermal efficiency is defined as
where:
Carnot's theorem sets essential limitations on the yield of a cyclic heat engine such as steam engines or internal combustion engines: they can extract only a certain proportion of mechanical energy from the heat of the working fluid; this maximal amount is realized by the ideal Carnot heat engine. |
|||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Carnot's_theorem_(thermodynamics)". A list of authors is available in Wikipedia. |





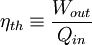 ,
,