To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Conjugate acidWithin the Brønsted-Lowry (protonic) theory of acids and bases, a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases a proton in the backward chemical reaction. Thus, the term acid. The base produced, X−, is called the conjugate base and it absorbs a proton in the backward chemical reaction. In aqueous solution, the chemical reaction involved is of the form
Product highlightThis principle is discussed in detail in the article on acid-base reaction theories. The conjugate base of a weak acid is a strong base, and the conjugate base of a strong acid is a weak base, and vice versa. Tabulated below are several examples of conjugate acid-base pairs. Acid strength decreases and base strength increases down the table. (The dissociation reaction reaches equilibrium further to the right, with more X− produced.)
See also
|
||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Conjugate_acid". A list of authors is available in Wikipedia. |


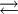 X− + H3O+
X− + H3O+





