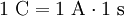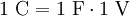To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
CoulombThe coulomb (symbol: C) is the SI unit of electric charge. It is named after Charles-Augustin de Coulomb. Product highlight
Definition1 coulomb is the amount of electric charge transported by a current of 1 ampere in 1 second. It can also be defined in terms of capacitance and voltage, where one coulomb is defined as one farad of capacitance times one volt of electric potential difference: ExplanationIn principle, the coulomb could be defined in terms of the charge of an electron or elementary charge. Since the values of the Josephson (CIPM (1988) Recommendation 1, PV 56; 19) and von Klitzing (CIPM (1988), Recommendation 2, PV 56; 20) constants have been given conventional values (KJ ≡ 4.835 979×1014 Hz/V and RK ≡ 2.581 280 7×104 Ω), it is possible to combine these values to form an alternative (not yet official) definition of the coulomb. A coulomb is then equal to exactly 6.241 509 629 152 65×1018 elementary charges. Combined with the present definition of the ampere, this proposed definition would make the kilogram a derived unit.[citation needed] If two point charges of +1 C are held one meter away from each other, the repulsive force they will feel is given by Coulomb's Law as 8.988×109 N [1]. This is roughly equal to the gravitational force of 900,000 metric tons of mass at the surface of the Earth; in everyday terms, it's enough force to accelerate an Airbus A380 airplane up to a final speed of 76,857 km/h in 1 second. In everyday life, most things don't have a large surplus of charge! Historical noteThe ampere was historically a derived unit—being defined as 1 coulomb per second. Therefore the coulomb, rather than the ampere, was the SI base electrical unit. In 1960 the SI system made the ampere the base unit. [1] SI multiples
Conversions
See also
References1.Kowalski, Ludwik, "A Short History of the SI Units in Electricity", pp. 97-99 vol 24, The Physics Teacher, Feb 1986 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Coulomb". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||