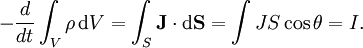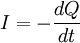To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electric charge
Electric charge is a fundamental conserved property of some subatomic particles, which determines their electromagnetic interaction. Electrically charged matter is influenced by, and produces, electromagnetic fields. The interaction between a moving charge and an electromagnetic field is the source of the electromagnetic force, which is one of the four fundamental forces. Electric charge is a characteristic of some subatomic particles, and is quantized when expressed as a multiple of the so-called elementary charge e. Electrons by convention have a charge of -1, while protons have the opposite charge of +1. Quarks have a fractional charge of −1/3 or +2/3. The antiparticle equivalents of these have the opposite charge. There are other charged particles. In general, same-sign charged particles repel one another, while different-sign charged particles attract. This is expressed quantitatively in Coulomb's law, which states the magnitude of the repelling force is proportional to the product of the two charges, and weakens proportionately to the square of the distance. The electric charge of a macroscopic object is the sum of the electric charges of its constituent particles. Often, the net electric charge is zero, since naturally the number of electrons in every atom is equal to the number of the protons, so their charges cancel out. Situations in which the net charge is non-zero are often referred to as static electricity. Furthermore, even when the net charge is zero, it can be distributed non-uniformly (e.g., due to an external electric field), and then the material is said to be polarized, and the charge related to the polarization is known as bound charge (while the excess charge brought from outside is called free charge). An ordered motion of charged particles in a particular direction (in metals, these are the electrons) is known as electric current. The discrete nature of electric charge was proposed by Michael Faraday in his electrolysis experiments, then directly demonstrated by Robert Millikan in his oil-drop experiment. The SI unit for quantity of electricity or electric charge is the coulomb, which represents approximately 6.24 × 1018 elementary charges (the charge on a single electron or proton). The coulomb is defined as the quantity of charge that has passed through the cross-section of an electrical conductor carrying one ampere within one second. The symbol Q is often used to denote a quantity of electricity or charge. The quantity of electric charge can be directly measured with an electrometer, or indirectly measured with a ballistic galvanometer. Formally, a measure of charge should be a multiple of the elementary charge e (charge is quantized), but since it is an average, macroscopic quantity, many orders of magnitude larger than a single elementary charge, it can effectively take on any real value. Furthermore, in some contexts it is meaningful to speak of fractions of a charge; e.g. in the charging of a capacitor. Product highlight
HistoryAs reported by the Ancient Greek philosopher Thales of Miletus around 600 BC, charge (or electricity) could be accumulated by rubbing fur on various substances, such as amber. The Greeks noted that the charged amber buttons could attract light objects such as hair. They also noted that if they rubbed the amber for long enough, they could even get a spark to jump. This property derives from the triboelectric effect. In 1600 the English scientist William Gilbert returned to the subject in De Magnete, and coined the New Latin word electricus from ηλεκτρον (elektron), the Greek word for "amber", which soon gave rise to the English words "electric" and "electricity." He was followed in 1660 by Otto von Guericke, who invented what was probably the first electrostatic generator. Other European pioneers were Robert Boyle, who in 1675 stated that electric attraction and repulsion can act across a vacuum; Stephen Gray, who in 1729 classified materials as conductors and insulators; and C. F. du Fay, who proposed in 1733 [2] that electricity came in two varieties which cancelled each other, and expressed this in terms of a two-fluid theory. When glass was rubbed with silk, du Fay said that the glass was charged with vitreous electricity, and when amber was rubbed with fur, the amber was said to be charged with resinous electricity. In 1839 Michael Faraday showed that the apparent division between static electricity, current electricity and bioelectricity was incorrect, and all were a consequence of the behavior of a single kind of electricity appearing in opposite polarities. It is arbitrary which polarity you call positive and which you call negative. Positive charge can be defined as the charge left on a glass rod after being rubbed with silk.[1] One of the foremost experts on electricity in the 18th century was Benjamin Franklin, who argued in favour of a one-fluid theory of electricity. Franklin imagined electricity as being a type of invisible fluid present in all matter; for example he believed that it was the glass in a Leyden jar that held the accumulated charge. He posited that rubbing insulating surfaces together caused this fluid to change location, and that a flow of this fluid constitutes an electric current. He also posited that when matter contained too little of the fluid it was "negatively" charged, and when it had an excess it was "positively" charged. Arbitrarily (or for a reason that was not recorded) he identified the term "positive" with vitreous electricity and "negative" with resinous electricity. William Watson arrived at the same explanation at about the same time. We now know that the Franklin/Watson model was fundamentally correct. There is only one kind of electrical charge, and only one variable is required to keep track of the amount of charge.[2] On the other hand, just knowing the charge is not a complete description of the situation. Matter is composed of several kinds of electrically charged particles, and these particles have many properties, not just charge. The most common charge carriers are the positively charged proton and the negatively charged electron. The movement of any of these charged particles constitutes an electric current. In many situations, it suffices to speak of the conventional current without regard to whether it is carried by positive charges moving in the direction of the conventional current and/or by negative charges moving in the opposite direction. This macroscopic viewpoint is an approximation that simplifies electromagnetic concepts and calculations. At the opposite extreme, if one looks at the microscopic situation, one sees there are many ways of carrying an electric current, including: a flow of electrons; a flow of electron "holes" which act like positive particles; and both negative and positive particles (ions or other charged particles) flowing in opposite directions in an electrolytic solution or a plasma). Beware that in the common and important case of metallic wires, the direction of the conventional current is opposite to the drift velocity of the actual charge carriers, i.e. the electrons. This is a source of confusion for beginners. PropertiesAside from the properties described in articles about electromagnetism, charge is a relativistic invariant. This means that any particle that has charge q, no matter how fast it goes, always has charge q. This property has been experimentally verified by showing that the charge of one helium nucleus (two protons and two neutrons bound together in a nucleus and moving around at high speeds) is the same as two deuterium nuclei (one proton and one neutron bound together, but moving much more slowly than they would if they were in a helium nucleus). Conservation of chargeThe total electric charge of an isolated system remains constant regardless of changes within the system itself. This law is inherent to all processes known to physics and can be derived in a local form from gauge invariance of the wave function. The conservation of charge results in the charge-current continuity equation. More generally, the net change in charge density ρ within a volume of integration V is equal to the area integral over the current density J on the surface of the area S, which is in turn equal to the net current I: Thus, the conservation of electric charge, as expressed by the continuity equation, gives the result: where I is the net outward current through a closed surface and Q is the electric charge contained within the volume defined by the surface. See also
References and External links
|
||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electric_charge". A list of authors is available in Wikipedia. |