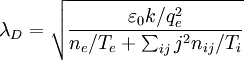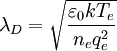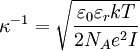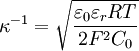To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Debye lengthThe notion of Debye length plays an important role in plasma physics, electrolytes and colloids (DLVO theory). Product highlightIn plasma physics, the Debye length, named after the Dutch physical chemist Peter Debye, is the scale over which mobile charge carriers (e.g. electrons) screen out electric fields in plasmas and other conductors. In other words, the Debye length is the distance over which significant charge separation can occur. A Debye sphere is a volume whose radius is the Debye length, in which there is a sphere of influence, and outside of which charges are screened. In space plasmas where the electron density is relatively low, the Debye length may reach macroscopic values, such as in the magnetosphere, solar wind, interstellar medium and intergalactic medium (see table):
http://www.pma.caltech.edu/Courses/ph136/yr2002/ Hannes Alfven pointed out that: "In a low density plasma, localized space charge regions may build up large potential drops over distances of the order of some tens of the Debye lengths. Such regions have been called electric double layers. An electric double layer is the simplest space charge distribution that gives a potential drop in the layer and a vanishing electric field on each side of the layer. In the laboratory, double layers have been studied for half a century, but their importance in cosmic plasmas has not been generally recognized.". Debye length in a plasmaIn a plasma, the Debye length is where
The ion term is often dropped, giving although this is only valid when the ions are much colder than the electrons. Debye length in an electrolyteIn an electrolyte or a colloidal dispersion, the Debye length is usually denoted with symbol κ-1 where
or when the solute is mono-monovalent and symmetrical, where
Alternatively, where
For water at room temperature, λB ≈ 0.7 nm. References
Categories: Colloidal chemistry | Plasma physics | Electrochemistry |
|||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Debye_length". A list of authors is available in Wikipedia. |