To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electronic densityIn quantum mechanics, and in particular in quantum chemistry, the electronic density ρ corresponding to an N-electron wavefunction Ψ(N) is the one-electron function given by Product highlightIn the case Ψ(N) is a Slater determinant made of N spin orbitals The two-electron electronic density is given by Those quantities are particularly important in the context of density functional theory. The coordinates x used here are the spin-spatial coordinates. Categories: Atomic physics | Quantum chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electronic_density". A list of authors is available in Wikipedia. |


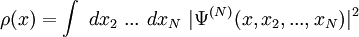



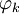 :
: