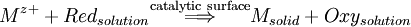To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
ElectroplatingElectroplating is the process of using electrical current to reduce metal cations in a solution and coat a conductive object with a thin layer of metal. The primary application of electroplating deposits a layer of a metal having some desired property (e.g., abrasion and wear resistance, corrosion protection, lubricity, improvement of aesthetic qualities, etc.) onto a surface lacking that property. Another application uses electroplating to build up thickness on undersized parts. The process used in electroplating is called electrodeposition. It is analogous to a galvanic cell acting in reverse. The part to be plated is the cathode of the circuit. In one technique, the anode is made of the metal to be plated on the part. Both components are immersed in a solution called an "Electrolyte" containing one or more dissolved metal salts as well as other ions that permit the flow of electricity. A rectifier supplies a direct current to the cathode causing the metal ions in the electrolyte solution to lose their charge and plate out on the cathode. As the electrical current flows through the circuit, the anode slowly dissolves and replenishes the ions in the bath.[1] Other electroplating processes may use a nonconsumable anode such as lead. In these techniques, ions of the metal to be plated must be periodically replenished in the bath as they are drawn out of the solution.[2] Product highlight
ProcessThe anode and cathode in the electroplating cell are connected to an external supply of direct current, a battery or, more commonly, a rectifier. The anode is connected to the positive terminal of the supply, and the cathode (article to be plated) is connected to the negative terminal. When the external power supply is switched on, the metal at the anode is oxidized from the zero valence state to form cations with a positive charge. These cations associate with the anions in the solution. The cation are reduced at the cathode to deposit in the metallic, zero valence state. Example: In an acid solution, Copper is oxidized from an anode to Cu2+ by losing two electrons. The Cu2+ associates with the anion SO42- in the solution to form copper sulfate. At the cathode, the Cu2+ is reduced to metallic Cu by gaining two electrons. The result is the effective transfer of Cu from the anode source to a plate covering the cathode. The plating is most commonly a single metallic element, not an alloy. However, some alloys can be electrodeposited, notably brass and solder. Many plating baths include cyanides of other metals (e.g., potassium cyanide) in addition to cyanides of the metal to be deposited. These free cyanides facilitate anode corrosion, help to maintain a constant metal ion level and contribute to conductivity. Additionally, non-metal chemicals such as carbonates and phosphates may be added to increase conductivity. When plating is not desired on certain areas, stop-offs are applied to prevent the bath from coming in contact with the substrate. Typical stop-offs include tape, foil, lacquers, and waxes.[3] StrikeInitially, a special plating deposit called a "strike" or "flash" may be used to form a very thin (typically less than 0.5 mil thick) plating with high quality and good adherence to the substrate. This serves as a foundation for subsequent plating processes. A strike uses a high current density and a bath with a low ion concentration. The process is slow, so more efficient plating processes are used once the desired strike thickness is obtained. The striking method is also used in combination with the plating of different metals. If it is desirable to plate one type of deposit onto a metal to improve corrosion resistance but this metal has inherently poor adhesion to the substrate, a strike can be first deposited that is compatible with both. One example of this situation is the poor adhesion of electrolytic nickel on zinc alloys, in which case a copper strike is used, which has good adherence to both.[4] Current densityThe current density (amperage of the electroplating current divided by the surface area of the part) in this process strongly influences the deposition rate, plating adherence, and plating quality. This density can vary over the surface of a part, as outside surfaces will tend to have a higher current density than inside surfaces (e.g., holes, bores, etc.). The higher the current density, the faster the deposition rate will be, although there is a practical limit enforced by poor adhesion and plating quality when the deposition rate is too high. While most plating cells use a continuous direct current, some employ a cycle of 8–15 seconds on followed by 1–3 seconds off. This allows high current densities to be used while still producing a quality deposit. In order to deal with the uneven plating rates that result from high current densities, the current is even sometimes reversed, causing some of the plating from the thicker sections to re-enter the solution. In effect, this allows the "valleys" to be filled without over-plating the "peaks." This is common on rough parts or when a bright finish is required.[5] Brush electroplatingA closely-related process is brush electroplating, in which localized areas or entire items are plated using a brush saturated with plating solution. The brush, typically a stainless steel body wrapped with a cloth material that both holds the plating solution and prevents direct contact with the item being plated, is connected to the positive side of a low voltage direct-current power source, and the item to be plated connected to the negative. The operator dips the brush in plating solution then applies it to the item, moving the brush continually to get an even distribution of the plating material. The brush acts as the anode, but typically does not contribute any plating material, although sometimes the brush is made from or contains the plating material in order to extend the life of the plating solution. Brush electroplating has several advantages over tank plating, including portability, ability to plate items that for some reason can't be tank plated (one application was the plating of portions of very large decorative support columns in a building restoration), low or no masking requirements, and comparatively low plating solution volume requirements. Disadvantages compared to tank plating can include greater operator involvement (tank plating can frequently be done with minimal attention), and inability to achieve as great a plate thickness. Electroless depositionUsually an electrolytic cell (consisting of two electrodes, electrolyte, and and external source of current) is used for electrodeposition. In contrast, an electroless deposition process uses only one electrode and no external source of electrical current. However, the solution for the electroless process needs to contains a reducing agent so that the electrode reaction has the form: For example, an electroless process is used for electroless nickel plating. CleanlinessCleanliness is essential to successful electroplating, since molecular layers of oil can prevent adhesion of the coating. ASTM B322 is a standard guide for cleaning metals prior to electroplating. Cleaning processes include solvent cleaning, hot alkaline detergent cleaning, electrocleaning, and acid etch. The most common industrial test for cleanliness is the waterbreak test, in which the surface is thoroughly rinsed and held vertical. Hydrophobic contaminants such as oils cause the water to bead and break up, allowing the water to drain rapidly. Perfectly clean metal surfaces are hydrophilic and will retain an unbroken sheet of water that does not bead up or drain off. ASTM F22 describes a version this test. This test does not detect hydrophilic contaminants, but the electroplating process can displace these easily since the solutions are water-based. Surfactants such as soap reduce the sensitivity of the test, so these must be thoroughly rinsed off. HistoryModern electrochemistry was invented by Italian chemist Luigi V. Brugnatelli in 1805. Brugnatelli used his colleague Alessandro Volta's invention of five years earlier, the voltaic pile, to facilitate the first electrodeposition. Unfortunately, Brugnatelli's inventions were repressed by the French Academy of Sciences and did not become used in general industry for the following thirty years. By 1839, scientists in Britain and Russia had independently devised metal deposition processes similar to Brugnatelli's for the copper electroplating of printing press plates. Soon after, John Wright of Birmingham, England discovered that potassium cyanide was a suitable electrolyte for gold and silver electroplating. Wright's associates, George Elkington and Henry Elkington were awarded the first patents for electroplating in 1840. These two then founded the electroplating industry in Birmingham, England from where it spread around the world. As the science of electrochemistry grew, its relationship to the electroplating process became understood and other types of non-decorative metal electroplating processes were developed. Commercial electroplating of nickel, brass, tin, and zinc were developed by the 1850s. Electroplating baths and equipment based on the patents of the Elkingtons were scaled up to accommodate the plating of numerous large scale objects and for specific manufacturing and engineering applications. The plating industry received a big boost from the advent of the development of electric generators in the late 1800s. With the higher currents available metal machine components, hardware, and automotive parts requiring corrosion protection and enhanced wear properties, along with better appearance, could be processed in bulk. The two World Wars and the growing aviation industry gave impetus to further developments and refinements including such processes as, hard chromium plating, bronze alloy plating, sulfamate nickel plating, along with numerous other plating processes. Plating equipment evolved from manually operated tar-lined wooden tanks to automated equipment, capable of processing thousands of pounds per hour of parts. One of American physicist Richard Feynman's first projects was to develop technology for electroplating metal onto plastic. Feynman successfully developed this technology, allowing his employer to keep commercial promises he had made but could not have fulfilled otherwise.[6] Electroplating is one of the three processes that form the LIGA-process used to manufacture MEMS devices. See also
Notes and references
Categories: Coatings | Chemical processes | Corrosion prevention | Metal plating |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electroplating". A list of authors is available in Wikipedia. |