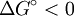To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Exergonic reactionAn exergonic reaction is a chemical reaction where the variation of Gibbs free energy is negative [1]. This tells us the direction that the reaction will follow. At constant temperature, constant pressure an exergonic reaction is signified by condition: Product highlightwhich describes a chemical reaction that releases energy in the form of heat, light, etc. Exergonic reactions are a form of exergonic processes in general or spontaneous processes and the opposite is called an endergonic reaction. Exergonic reactions are said to occur spontaneously but this does not imply that the reaction will take place unconstrained. For instance the reaction between hydrogen and oxygen is very slow and not observed in absence of a suitable catalyst. It has been suggested to replace the term spontaneous with eager [2]. Key points
References |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Exergonic_reaction". A list of authors is available in Wikipedia. |