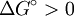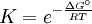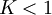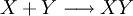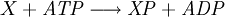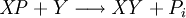To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Endergonic reactionIn chemical thermodynamics, an endergonic reaction (also called an unfavorable reaction or a nonspontaneous reaction) is a chemical reaction in which the standard change in free energy is positive, and energy is absorbed. Under constant temperature and constant pressure conditions, this means that the change in the standard Gibbs free energy would be positive for the reaction at standard state (ie at standard pressure (1 bar), and standard concentrations (1 molar) of all the reagents). Product highlight
Equilibrium constantThe equilibrium constant for the reaction is related to ΔG° by the relation: where T the absolute temperature and R is the gas constant. A positive value of ΔG° therefore implies so that starting from molar stoichiometric quantities such a reaction would move backwards toward equilibrium, not forwards. Nevertheless, endergonic reactions are quite common in nature, especially in biochemistry and physiology. Examples of endergonic reactions in cells include protein synthesis, and the Na+/K+ pump which drives nerve conduction and muscle contraction. Making Endergonic reactions happenEndergonic reactions can be achieved if they are either pulled or pushed by an exergonic (stability increasing, negative change in Free Energy) process. PullReagents can be pulled through an endergonic reaction, if the reaction products are cleared rapidly by a subsequent exergonic reaction. The concentration of the products of the endergonic reaction thus always remains low, so the reaction can proceed. A classic example of this might be the first stage of a reaction which proceeds via a transition state. The process of getting to the top of the activation energy barrier to the transition state is endergonic. However, the reaction can proceed because having reached the transition state, it rapidly evolves via an exergonic process to the more stable final products. PushEndergonic reactions can be pushed by coupling them to another reaction which is strongly exergonic, through a shared intermediate. This is often how biological reactions proceed. For example, on its own the reaction may be too endergonic to occur. However it may be possible to make it occur by coupling it to a strongly exergonic reaction – such as, very often, the decomposition of ATP into ADP and inorganic phosphate ions, ATP → ADP + Pi, so that This kind of reaction, with the ATP decomposition supplying the free energy needed to make an endergonic reaction occur, is so common in cell biochemistry that ATP is often called the "universal energy currency" of all living organisms. See also
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Endergonic_reaction". A list of authors is available in Wikipedia. |