To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Gallium trichloride
Gallium trichloride is the chemical compound with the formula GaCl3. Solid gallium trichloride exists as a dimer with the formula Ga2Cl6.[1] It is colourless and soluble in virtually all solvents, which is unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.[2] As a Lewis acid, GaCl3 is milder than aluminium trichloride. Gallium(III) is easier to reduce than Al(III), so the chemistry of reduced gallium compounds is more extensive than for aluminium. Ga2Cl4 is known whereas the corresponding Al2Cl4 is not. The coordination chemistry of Ga(III) and Fe(III) are similar, and gallium(III) compounds have been used as diamagnetic analogues of ferric compounds. Product highlight
PreparationGallium trichloride can be prepared from the elements, heating gallium metal in a stream of chlorine, and purifying the product by sublimation under vacuum.[3][4] StructureThe dimeric structure involving two chlorine bridging atoms with the gallium atoms essentially tetrahedrally coordinated by chlorine is surprising, as the chlorides of the two members of group 13 above and below gallium, AlCl3 and InCl3 both contain 6 coordinate metal atoms in a three dimensional structure. As a consequence of its structure where there are no significant lattice forces, gallium trichloride has the lowest melting point of all of the aluminium, gallium and indium trihalides. The formula of Ga2Cl6 is often written as Ga2(μ-Cl)2Cl4. In the gas phase the dimers dissociate to trigonal planar monomers. ComplexesGallium is the lightest member of group 13 to have a full d shell, (gallium has the electronic configuration Ar 3d10 4s2 4p1) below the valence electrons that could take part in d-π bonding with ligands. This in terms, hard and soft acid-base theory allows gallium(III) to behave as "soft" acid. The strength of the bonds between gallium halides and ligands have been extensively studied. What emerges is:
With a chloride ion as ligand the tetrahedral GaCl4− ion is produced, the 6 coordinate GaCl63− cannot be made. Compounds like KGa2Cl7 that have a chloride bridged anion are known[5] In a molten mixture of KCl and GaCl3, the following equilibrium exists:
Use in detection of solar neutrinos110 tons of gallium trichloride solution has been used in GALLEX experiment performed by Laboratori Nazionali del Gran Sasso in Italy to detect solar neutrinos. In this experiment, isotope germanium-71 is produced and being measured.[6] See alsoReferences
Categories: Inorganic compounds | Gallium compounds | Chlorides | Metal halides |
|||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Gallium_trichloride". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||





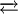 Ga2Cl7− + Cl−
Ga2Cl7− + Cl−


