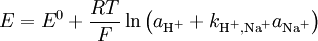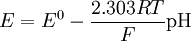To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Glass electrodeA glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. Product highlight
TypesAlmost all commercial electrodes responds to single charged ions, like H+, Na+, Ag+. The most common glass electrode is the pH-electrode. Only a few chalcogenide glass electrodes are sensitive to double-charged ions, like Pb2+, Cd2+ and some other. There are two main glass-forming systems:
Interfering ionsBecause of the ion-exchange nature of the glass membrane, it is possible for some other ions to concurrently interact with ion-exchange centers of the glass and to distort the linear dependence of the measured electrode potential on pH or other electrode function. In some cases it is possible to change the electrode function from one ion to another. For example, some silicate pNa electrodes can be changed to pAg function by soaking in a silver salt solution. Interference effects are commonly described the semiempirical Nicolsky-Eisenman equation[1], an extension to the Nernst equation. It is given by where E is the emf, E0 the standard electrode potential, z the ionic valency including the sign, a the activity, i the ion of interest, j the interfering ions and kij is the selectivity coefficient. The smaller the selectivity coefficient, the less is the interference by j. To see the interfering effect of Na+ to a pH-electrode:
Range of a pH glass electrodeThe pH range at constant concentration can be divided into 3 parts:
There are different types of pH glass electrode, some of them have improved characteristics for working in alkaline or vice versa, acidic medium. But almost all electrodes have sufficient properties for working in the most popular pH range from pH=1 till pH=12. Special electrodes should be used only for working in aggressive conditions. Most of text written above is also correct for any ion-exchange electrodes. Construction
A typical modern pH probe is a combination electrode, which combines both the glass and reference electrodes into one body. The bottom of a pH electrode balloons out into a round thin glass bulb. The pH electrode is best thought of as a tube within a tube. The inside most tube (the inner tube) contains an unchanging saturated KCl and a 0.1M HCl solution. Also inside the inner tube is the cathode terminus of the reference probe. The anodic terminus wraps itself around the outside of the inner tube and ends with the same sort of reference probe as was on the inside of the inner tube. Both the inner tube and the outer tube contain a reference solution but only the outer tube has contact with the solution on the outside of the pH probe by way of a porous plug that serves as a salt bridge. This device is essentially a galvanic cell. The reference end is essentially the inner tube of the pH meter, which for obvious reasons cannot lose ions to the surrounding environment (as a reference is good only so long as it stays static through the duration of the measurement). The outer tube contains the medium, which is allowed to mix with the outside environment (and as a consequence this tube must be replenished with a solution of KCl due to ion loss and evaporation). The measuring part of the electrode, the glass bulb on the bottom, is coated both inside and out with a ~10nm layer of a hydrated gel. These two layers are separated by a layer of dry glass. The silica glass structure (that is, the conformation of its atomic structure) is shaped in such a way that it allows Na+ ions some mobility. The metal cations (Na+) in the hydrated gel diffuse out of the glass and into solution while H+ from solution can diffuse into the hydrated gel. It is the hydrated gel, which makes the pH electrode an ion selective electrode. H+ does not cross through the glass membrane of the pH electrode, it is the Na+ which crosses and allows for a change in free energy. When an ion diffuses from a region of activity to another region of activity, there is a free energy change and this is what the pH meter actually measures. The hydrated gel membrane is connected by Na+ transport and thus the concentration of H+ on the outside of the membrane is 'relayed' to the inside of the membrane by Na+. All glass pH electrodes have extremely high electric resistance from 50 to 500 MOhm. Therefore, the glass electrode can be used only with a high impedance measuring device like a pH meter, or a more universal measuring device - ionometer. StorageBetween measurements any glass and membrane electrodes should be kept in the solution of its own ion (Ex. pH glass electrode should be kept in 0.1M HCl or 0.1M H2SO4). It is necessary to prevent the glass membrane from drying out. See also
References
Categories: Electrodes | Electrochemistry |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Glass_electrode". A list of authors is available in Wikipedia. |





![E=E^0 + \frac{RT}{z_iF} \ln \left [ a_i + \sum_{j} \left ( k_{ij}a_j^{z_i/z_j} \right ) \right ]](images/math/f/5/0/f50d82a82e3031605ab7579a0f806f24.png)