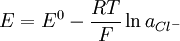To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Silver chloride electrodeA silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For example, it is usually the internal reference electrode in pH meters. As another example, the silver chloride electrode is the most commonly used reference electrode for testing cathodic protection corrosion control systems in sea water environments. The electrode functions as a redox electrode and the reaction is between the silver metal (Ag) and its salt — silver chloride (AgCl, also called silver(I) chloride). The corresponding equations can be presented as follows:
or an overall reaction can be written:
This reaction characterized by fast electrode kinetics, meaning that a sufficiently high current can be passed through the electrode with the 100% efficiency of the redox reaction (dissolution of the metal or cathodic deposition of the silver-ions). The reaction has been proved to obey these equations in solutions with pH’s of between 0 and 13.5. The Nernst equation below shows the dependence of the potential of the silver-silver(I) chloride electrode on the activity or effective concentration of chloride-ions: The standard electrode potential E0 against standard hydrogen electrode is 0.230V ± 10mV. The potential is however very sensitive to traces of bromide ions which make it more negative. (The more exact standard potential given by an IUPAC review paper is O.22249 V, with a standard deviation of 0.13 mV at 25 °C [1].) Product highlight
ApplicationsCommercial reference electrodes consist of a plastic tube electrode body. The electrode is a silver wire that is coated with a thin layer of silver chloride, either by electroplating or by dipping the wire in molten silver chloride. A porous plug on one end allows contact between the field environment with the silver chloride electrolyte. An insulated lead wire connects the silver rod with measuring instruments. A voltmeter negative lead is connected to the test wire. The reference electrode contains potassium chloride to stabilize the silver chloride concentration. The potential of a silver:silver chloride reference electrode with respect to the standard hydrogen electrode depends on the electrolyte composition.
The electrode has many features making is suitable for use in the field:
They are usually manufactured with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 1 mol/kg potassium chloride. As noted above, changing the electrolyte concentration changes the electrode potential. Silver chloride is slightly soluble in strong potassium chloride solutions, so it is sometimes recommended the potassium chloride be saturated with silver chloride to avoid stripping the silver chloride off the silver wire. Elevated temperature applicationWhen appropriately constructed, the silver chloride electrode can be used up to 300 °C. The standard potential (i.e., the potential when the chloride activity is 1 mol/kg) of the silver chloride electrode is a function of temperature as follows[4]:
Bard et al.[5] give the following correlations for the standard potential of the silver chloride electrode as a function of temperature (where t is temperature in °C): E0(V) = 0.23695 - 4.8564x10-4t - 3.4205x10-6t2 - 5.869 x 10-9t3 for 0 < t < 95 °C. The same authors also give the fit to the high-temperature potential, but it appears to contain a typographic error. The corrected fit, which reproduced the data in the table above is:[6] E0(V) = 0.23735 - 5.3783x10-4t - 2.3728x10-6t2 - 2.2671x10-9(t+273) for 25 < t < 275 °C. An extrapolation to 300 °C gives E0 of -0.138 V. See also
For use in soil they are usually manufactured with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 1 M potassium chloride. In seawater or chlorinated potable water they are usually directly immersed with no separate electrolyte.As noted above, changing the electrolyte concentration changes the electrode potential. Silver chloride is slightly soluble in strong potassium chloride solutions, so it is sometimes recommended that the potassium chloride be saturated with silver chloride. References
Categories: Electrodes | Electrochemistry |
||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Silver_chloride_electrode". A list of authors is available in Wikipedia. |