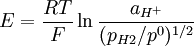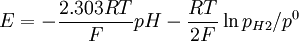To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Standard hydrogen electrodeThe standard hydrogen electrode (abbreviated SHE), also called normal hydrogen electrode (NHE), is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be 4.44 ± 0.02 V at 25 °C, but to form a basis for comparison with all other electrode reactions, Hydrogen's standard electrode potential (E0) is declared to be zero at all temperatures[1]. Potentials of any other electrodes are compared with that of the standard hydrogen electrode at the same temperature. Hydrogen electrode is based on the redox half cell:
This redox reaction occurs at platinized platinum electrode. The Nernst equation should be written as: or where:
Product highlight
Why platinum?The choice of platinum for the hydrogen electrode is due to several factors:
The surface of platinum is platinized (i.e., covered with platinum black) because of:
Nevertheless, other metals can be used for building electrodes with a similar function, for example, palladium-hydrogen electrode.
InterferenceBecause of the high adsorption activity of the platinized platinum electrode, it's very important to protect electrode surface and solution for the presence of organic substances as well as oxygen of atmosphere. Construction
The scheme of the standard hydrogen electrode:
See alsoReferencesCategories: Electrodes | Electrochemistry | Hydrogen technologies |
|||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Standard_hydrogen_electrode". A list of authors is available in Wikipedia. |