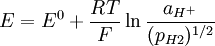To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Palladium-hydrogen electrodeThe Palladium-Hydrogen electrode (Abbreviation: Pd/H2) is one of the common reference electrodes used in electrochemical study. Most of its characteristics are similar to the standard hydrogen electrode (with platinum). But palladium has one significant feature - the capability to absorb (dissolve into itself) molecular hydrogen. Product highlightTwo phases can coexist in palladium when hydrogen is absorbed:
The electrochemical behaviour of a palladium electrode in equilibrium with H3O+ ions in solution parallels the behaviour of palladium with molecular hydrogen
When palladium is electrochemically charged by hydrogen, the existence of two phases is manifested by a constant potential of aprox. +50 mV vs reversible hydrogen electrode. This potential is independent of the amount of hydrogen absorbed over a wide range. This property has been utilized in the construction of a palladium/hydrogen reference electrode. The main feature of such electrode is an absence of non-stop bubbling of molecular hydrogen through the solution as it is absolutely necessary for the standard hydrogen electrode. References
Categories: Electrodes | Electrochemistry | Palladium | Hydrogen technologies |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Palladium-hydrogen_electrode". A list of authors is available in Wikipedia. |