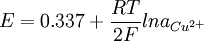To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Copper-copper(II) sulfate electrodeThe Copper-copper(II) sulfate electrode is a type of reference electrode, based on the redox reaction with participation of the metal (copper) and its salt - copper(II) sulfate. It is used for measuring electrochemical potential and is the most commonly used reference electrode for testing cathodic protection corrosion control systems. Product highlightThe corresponding equation can be presented as follow:
This reaction characterized by fast electrode kinetics, meaning that a sufficiently high current can be passed through the electrode with the 100% efficiency of the redox reaction (dissolution of the metal or cathodic deposition of the copper-ions). The Nernst equation below shows the dependence of the potential of the copper-copper(II) sulfate electrode on the activity or concentration copper-ions:
Commercial reference electrodes consist of a plastic tube holding the copper rod and saturated solution of copper sulfate. A porous plug on one end allows contact with the copper sulfate electrolyte. The copper rod protrudes out the tube. A voltmeter negative lead is connected to the copper rod. The potential of a copper copper sulfate electrode is +0.318 volt with respect to the standard hydrogen electrode. Applications
Categories: Electrodes | Electrochemistry | Corrosion prevention |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Copper-copper(II)_sulfate_electrode". A list of authors is available in Wikipedia. |