To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Copper coulometerThe copper coulometer is a one of the common application of the copper-copper(II) sulfate electrode. Such a coulometer consists of two identical copper electrodes immersed into the slightly acidic pH-buffered solution of the copper(II) sulfate. Passing of the current through the element leads to the anodic dissolution of the metal on anode and simultaneous deposition of the copper-ions on the cathode. These reactions have 100% efficiency over a wide range of the current densities. Amount of the quantity of electricity passed through the cell can be easily calculated by mass changes of any of the electrodes:
Product highlightwhere Q is the quantity of electricity (coulombs); Categories: Physical chemistry | Electrochemistry | Copper |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Copper_coulometer". A list of authors is available in Wikipedia. |


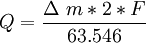 ,
,


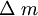 is the mass transported (gm); 63.546 is the
is the mass transported (gm); 63.546 is the 

