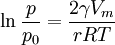To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Kelvin equationKelvin equation describes the change of vapour pressure over liquid curved with a radius r (for example, in a capillary or over a droplet). The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry. Where: p - actual vapour pressure Product highlightEquilibrium vapor pressure depends on droplet size. If p0 < p, then liquid evaporates from the droplets. If p0 >p , then the gas condenses onto the droplets… and they grow. As r increases, p decreases and the droplets grow into bulk liquid. If we now cool the vapour, then T decreases, but so does p0 . This means p/p0 increases as the liquid is cooled. We can treat γ and V as approximately fixed, which means that the critical radius r must also decrease. The further you supercool a vapour, the smaller the critical radius becomes. Ultimately it gets as small as a few molecules and the liquid undergoes homogeneous nucleation and growth. See alsoReferences
Categories: Surface chemistry | Physical chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Kelvin_equation". A list of authors is available in Wikipedia. |