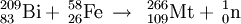To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Meitnerium
Meitnerium (pronounced /maɪtˈnɝiəm/), also called eka-iridium, is a chemical element in the periodic table that has the symbol Mt and atomic number 109. It is a synthetic element whose most stable isotope is Mt-276 with a half-life of 720 milliseconds. Product highlightHistoryMeitnerium was first synthesized on August 29, 1982 by a German research team led by Peter Armbruster and Gottfried Münzenberg at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung) in Darmstadt. The synthesis of this element demonstrated that nuclear fusion techniques could be used to make new, heavy nuclei. The name meitnerium was suggested in honor of the Austrian physicist and mathematician Lise Meitner, but there was an element naming controversy as to what the elements from 101 to 109 were to be called; thus IUPAC adopted unnilennium (/ˌjuːn Categories: Chemical elements | Transition metals |
|||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Meitnerium". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||