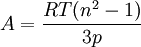To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Molar refractivityMolar refractivity is a measure of the volume occupied by an atom or group and is dependent on the temperature, the index of refraction, and the pressure. Product highlightOne form of the Lorentz-Lorenz formula (also known as the Clausius-Mossotti equation) gives the molar refractivity of a dilute gas as
where
In SI units, R has units of J mol-1 K-1, T has units K, n has no units, and p has units of Pa, so the units of A are m3 mol-1. Therefore, the molar refractivity is the volume of the substance (in cubic meters) taken up by each mole of that substance. |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Molar_refractivity". A list of authors is available in Wikipedia. |