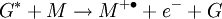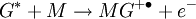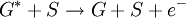To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Penning ionizationPenning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms and/or molecules.[1][2][3] The term Penning ionization refers to the interaction between a gas-phase excited-state atom or molecule G* and a target molecule M resulting in the formation of a radical molecular cation M+., an electron e- and a neutral gas molecule G. Product highlightReactionsAssociative Penning ionization can occur: Surface Penning ionization refers to the interaction of the excited-state gas with a surface S, resulting in the release of an electron. Penning ionization occurs when the target molecule has an ionization potential lower than the internal energy of the excited-state atom or molecule. The process was first reported by F.M. Penning in 1927. See alsoReferences
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Penning_ionization". A list of authors is available in Wikipedia. |