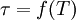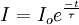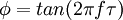To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Phosphor thermometry
Product highlight
Time Dependence of LuminescenceTypically a short duration ultraviolet lamp or laser source illuminates the phosphor coating which in turn luminesces visibly. When the illuminating source ceases, the luminescence will persist for a characteristic time, steadily decreasing. The time required for the brightness to decrease to
The intensity, I of the luminescence commonly decays exponentially as:
Where I0 is the initial intensity (or amplitude). The phosphors used may also be designated as "thermographic phosphors". The method is also referred to as fluorescence thermometry since it is also the case that similar materials in the form of glass, crystals, or even optical fibers will fluoresce and may be used as temperature sensors. Fiberoptic amplifiers are based on optical fibers doped with rare earths. Such fibers are useful for temperature measurement. If the excitation souce is periodic rather than pulsed, then the time response of the luminescence is correspondingly different. For instance, there is a phase difference between a sinusoidally varying light emitting diode (LED) signal of frequency f and the fluorescence that results. This is illustrated in the figure. The phase difference varies with decay time and hence temperature in the following way:
Temperature Dependence for Selected MaterialsThe right phosphor to use depends on the temperature range of interest, desired sensitivity, and other factors. The next two plots show the characteristic decay time versus temperature for several materials of use in the low to moderate temperature range and the high temperature range. Observations: 1. The oxysulfide materials represented there exhibit several different emission lines, each having a different temperature dependence. It is seen that substituting one rare-earth for another, in this instance changing La to Gd, shifts the temperature dependence. 2. The YAG:Cr material (Y3Al5O12:Cr3+) shows less sensiivity but covers a wider temperature range than the more sensitive materials. 3. Sometimes the decay time will be constant over a wide range before becoming temperature dependent at some threshold value. This is illustrated for the YVO4:Dy curve but also holds for several of the other curves as well (but not shown for clarity). 4. These curves are approximate. They may change somewhat depending on the fabrication process and the level of impurities. Sometimes manufacturers will add a second rare earth as a sensitizer. This may enhance the emission in some way for their purposes and may also alter the nature of the temperature dependence. Also, Ga is sometimes substituted for some of the Al in YAG, also altering the temperature dependence. 5. Non-exponential behaviour of the emission can be a factor. The emission of Dy phosphors is sometimes not easily modelled as a simple single exponential. Consequently, the value assigned to decay time will depend very much on the analysis method chosen. As dopant concentration increases, this non-exponential character often becomes more pronounced.
7. Particle Size is another important parameter. The decay time of a given phosphor will exhibit a change as a function of particle size. This is especially the case as the particle size decreases below a micrometre. 8. The data plotted here represents only a fraction of thermally sensitive luminescent materials that have been tested by various researchers. 9. The data illustrates the general conclusion that rare-earth and transition-metal oxides, vanadates, garnets, and phosphates can efficiently be made to fluoresce at high temperatures. See alsoCommercial firms involved in phosphor thermometry
Organizations involved in related research
References
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Phosphor_thermometry". A list of authors is available in Wikipedia. |





 of its original value is known as the decay time or lifetime and signified as
of its original value is known as the decay time or lifetime and signified as