To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Rutherford scatteringIn physics, Rutherford scattering is a phenomenon that was explained by Ernest Rutherford in 1911, and led to the development of the orbital theory of the atom. It is now exploited by the materials analytical technique Rutherford backscattering. Rutherford scattering is also sometimes referred to as Coulomb scattering because it relies on static electric (Coulomb) forces. A similar process probed the insides of nuclei in the 1960s, called deep inelastic scattering. The discovery was made by Hans Geiger and Ernest Marsden in 1911 when they performed the gold foil experiment under the direction of Rutherford, in which they fired a beam of alpha particles (helium nuclei) at layers of gold leaf only a few atoms thick. At the time of the experiment, the atom was thought to be analogous to a plum pudding (as proposed by J.J. Thomson), with the negative charges (the plums) found throughout a positive sphere (the pudding). If the plum-pudding model were correct, the positive “pudding”, being more spread out than in the current model of a concentrated nucleus, would not be able to exert such large coulombic forces, and the alpha particles should only be deflected by small angles as they pass through. However, the intriguing results showed that around 1 in 8000 alpha particles were deflected by very large angles (over 90°). From this, Rutherford concluded that the majority of the mass was concentrated in a minute, positively charged region (the nucleus) surrounded by electrons. When a (positive) alpha particle approached sufficiently close to the nucleus, it was repelled strongly enough to rebound at high angles. The small size of the nucleus explained the small number of alpha particles that were repelled in this way. Rutherford showed, using the method below, that the size of the nucleus was less than about 10−14 m (how much less than this size, Rutherford could not tell from this experiment alone; see more below on this problem of lowest possible size). Product highlight
Differential cross sectionAs derived by Rutherford in 1911, the differential cross section is All particles that go through the ring on the left end up somewhere in the ring on the right. Details of calculating maximal nuclear sizeFor head on collisions between alpha particles and the nucleus, all the kinetic energy ( Applying the inverse-square law between the charges on the electron and nucleus, one can write: 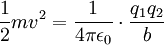 Rearranging: 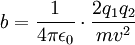 For an alpha particle:
Substituting these in gives the value of about 2.7×10−14 m. (The true radius is about 7.3×10−15 m.) The true radius of the nucleus is not recovered in these experiments because the alphas do not have enough energy to penetrate to more than 27 fm of the nuclear center, as noted, when the actual radius of gold is 7.3 fm. Rutherford realized this, and also realized that actual impact of the alphas on gold causing any force-deviation from that of the 1/r coulomb potential would change the form of his scattering curve at high scattering angles (the smallest impact parameters). This was not seen, indicating that the gold had not been "hit" so that Rutherford only knew the gold nucleus (or total of gold and alpha radius) was smaller than 27 fm (2.7×10−14 m) In 1919, a very similar experiment in Rutherford's laboratory showed departures from Coulombic scattering from different energy alphas on hydrogen nuclei, with a departure radius (indicating a true "collision" or change in force characteristics) occurring at about a calculated impact parameter or closest approach of 3.5 fm. Further investigations of alpha scattering on nitrogen and oxygen in Rutherford's laboratory convinced Chadwick and others by 1921 that at these scales and energies, forces which were other than simple coulomb repulsive forces were at work in close nuclear interactions. [1]. See alsoReferences
Categories: Scattering | Foundational quantum physics |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Rutherford_scattering". A list of authors is available in Wikipedia. |





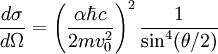
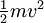 ) of the alpha particle is turned into potential energy and the particle is at rest. The distance from the centre of the alpha particle to the centre of the nucleus (b) at this point is a maximum value for the radius, if it is evident from the experiment that the particles have not hit the nucleus.
) of the alpha particle is turned into potential energy and the particle is at rest. The distance from the centre of the alpha particle to the centre of the nucleus (b) at this point is a maximum value for the radius, if it is evident from the experiment that the particles have not hit the nucleus.


