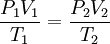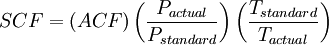To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
SCFMSCFM (Standard Cubic Feet per Minute) is the volumetric flow rate of a gas corrected to "standardized" conditions of temperature, pressure and relative humidity, thus representing a precise mass flow rate. However, great care must be taken, as the "standard" conditions vary between definitions and should therefore always be checked. Worldwide, the "standard" condition for pressure is variously defined as an absolute pressure of 101325 pascals, 1.0 bar (i.e., 100,000 pascals), 14.73 psia, or 14.696 psia and the "standard" temperature is variously defined as 68°F, 0°C, 15°C, 20°C or 25°C. The relative humidity (e.g., 36% or 0%) is also included in some definitions of standard conditions. There is, in fact, no universally accepted set of standard conditions. (See Standard conditions for temperature and pressure). The temperature variation is the most important. In Europe, the standard temperature is most commonly defined as 0°C (but not always). In the United States, the standard temperature is most commonly defined as 60°F or 70°F (but again not always). A variation in standard temperature can result in a significant volumetric variation for the same mass flow rate. For example, a mass flow rate of 1000 kg/hr of air at 1 atmosphere of absolute pressure is 455 SCFM when defined at 0°C (32°F) but 481 SCFM when defined at 60°F (15.56°C}. In countries using the SI metric system of unit, the term Normal Cubic Metre (Nm3) is very often used to denote gas volumes at some normalized or standard condition. Again, as noted above, there is no universally accepted set of normalized or standard conditions. Product highlight
ACFMActual Cubic Feet per Minute (ACFM) is the volume of gas flowing anywhere in a system, independent of its temperature and pressure. If the system were moving a gas at exactly the "standard" condition, then ACFM would equal SCFM. Unfortunately, this usually is not the case as the most important change between these two definitions is the pressure. To move a gas, a positive pressure or a vacuum must be created. When positive pressure is applied to a standard cubic foot of gas, it gets smaller. When a vacuum is applied to a standard cubic foot of gas, it expands. The volume of gas after it is pressurized or rarefied is referred to as its "actual" volume. SCF and ACF (for any gas) are related in accordance with the combined gas law:[1][2][3] Defining standard conditions by the subscript 1 and actual conditions by the subscript 2, then:[1][2][4] where P is in absolute pressure units and T is in absolute temperature units (i.e., either kelvins or degrees Rankine). To be very precise when the gas is air, then the above equation should include correcting for the difference between the relative humidity of the air at the standard and the actual temperature and pressure conditions.[5] In most cases of engineering design, the humidity correction for air is often quite small and hence often ignored. CFMCFM is an often confusing term because it has no single definition that applies to all instances. In the most basic sense, CFM means cubic feet per minute. Unfortunately, air is a compressible gas. To further confuse the issue, a centrifugal fan is a constant CFM device or a constant volume device. This means that, provided the fan speed remains constant, a centrifugal fan will pump a constant volume of air. This is not the same as pumping a constant mass of air. Again, the fan will pump the same volume, though not mass, at any other air density. This means that the air velocity in a system is the same even though mass flow rate through the fan is not. See also
References
Categories: Chemical engineering | Gases |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "SCFM". A list of authors is available in Wikipedia. |