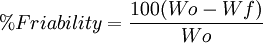To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Tablet and dose preparation, pharmaceutical preparationProduct highlight
Introduction to tabletsThe compressed tablet is the most popular dosage form in use today. About two-thirds of all prescriptions are dispensed as solid dosage forms, and half of these are compressed tablets. A tablet can be formulated to deliver an accurate dosage to a specific site; it is usually taken orally, but can be administered sublingually, rectally or intravaginally. Tablet formation represents the last stage in down-stream processing within the pharmaceutical industry. It is just one of the many forms that an oral drug can take such as syrups, elixirs, suspensions, and emulsions. It consists of an active pharmaceutical ingredient (A.P.I.) with biologically inert excipients in a compressed, solid form. Advantages and disadvantagesTablets are easy and convenient to use. They provide an accurately measured dosage in a convenient portable package; and can be designed to protect unstable medications or disguise unpalatable ingredients. Coatings can be coloured or stamped to aid tablet recognition. Manufacturing processes and techniques can provide tablets special properties; for example enteric coatings or sustained release formulations. Some drugs may be unsuitable for administration by the oral route. For example protein drugs such as insulin may be denatured by stomach acids; such drugs cannot be made into tablets. Some may be deactivated by the liver (the "first pass effect") making them unsuitable for oral use. However, drugs which can be taken sublingually bypass the liver and are less susceptible to the first pass effect. Bioavailability of some drugs may be low due to poor absorption from the gastric tract; such drugs may need to be given in very high doses or by injection. For drugs that need to have rapid onset, or have severe side effects the oral route may not be suitable. For example Salbutamol can have effects on the heart and circulation if taken orally; these effects are greatly reduced by inhaling smaller doses direct to the required site of action. Tablet propertiesTablets can be made in virtually any shape, although requirements of patients and tabletting machines mean that most are round, oval or capsule shaped. More unsusual shapes have been manufactured but patients find these harder to swallow, and they are more vulnerable to chipping or manufacturing problems. Tablet diameter and shape are determined a combination of a a set of punches and a die. This is called a station of tooling. The thickness is determined by the amount of tablet material and the position of the punches in relation to each other during compression. Once this is done, we can measure the corresponding pressure applied during compression. The shorter the distance between the punches, thickness, the greater the pressure applied during compression, and sometimes the harder the tablet. Tablets need to be hard enough that they don't break up in the bottle, yet friable enough that they disintegrate in the gastric tract. The tablet is composed of the Active Pharmaceutical Ingredient (that is the active drug) together with various excipients. These are biologically inert ingredients which either enhance the therapeutic effect or are necessary to construct the tablet. The filler or diluent (eg lactose or sorbitol)is a bulking agent, providing a quantity of material which can accurately be formed into a tablet. Binders eg methyl cellulose or gelatin) hold the ingredients together so that they can form a tablet. Lubricants (eg magnesium stearate or polyethylene glycol) are added to reduce the friction between the tablet and the punches and dies so that the tablet compression and ejection processes are smooth. Disintegrants (eg starch or cellulose) are used to promote wetting and swelling of the tablet so that it breaks up in the gastro intestinal tract; this is necessary to ensure dissolution of the API. Superdisintegrants are sometimes used to greatly speed up the disintegration of the tablet. Additional ingredients may also be added such as coloring agents, flavoring agents, and coating agents. Example of tablet composition
ManufacturingIn the tablet-pressing process, it is important that all ingredients be dry, powdered, and of uniform grain size as much as possible. The main guideline in manufacture is to ensure that the appropriate amount of active ingredient is equal in each tablet so ingredients should be well-mixed. Compressed tablets are exerted to great pressure in order to compact the material. If a sufficiently homogenous mix of the components cannot be obtained with simple mixing, the ingredients must be granulated prior to compression to assure an even distribution of the active compound in the final tablet. Two basic techniques are used to prepare powders for granulation into a tablet: wet granulation and dry granulation. Powders that can be mixed well and therefore do no require granulation can be compressed in to tablet through Direct Compression Direct compressionThis method is used when a group of ingredients can be blended and placed in a tablet press to make a tablet without any of the ingredients having to be changed. This is not very common because many tablets have active pharamaceutical ingredients which will not allow for direct compression due to their concentration or the excipients used in formulation are not conducive to direct compression. Granulation is the process of collecting particles together by creating bonds between them. There are several different methods of granulation. The most popular, which is used by over 70% of formulation in tablet manufacture is wet granulation. Dry granulation is another method used to form granules. Wet granulationWet granulation is a process of using a liquid binder or adhesive to the power mixture. The amount of liquid can be properly managed, and overwetting will cause the granules to be too hard and underwetting will cause the granules to be too soft and friable. Aqueous solutions have the advantage of being safer to deal with than solvents.
Water may be used as the liquid binder, but sometimes many actives are not compatible with water. Water mixed into the powder can form bonds between powder particles that are strong enough to lock them in together. However, once the water dries, the powders may fall apart and therefore might not be strong enough to create and hold a bond. Povidone also known as polyvinyl pyrrolidone (PVP) is one of the most commonly used pharmaceutical binders. PVP and a solvent are mixed with the powders to form a bond during the process, and the solvent evaporates. Once the solvent evaporates and powders have formed a densely held mass, then the granulation is milled which results in formation of granules Dry granulationThis process is used when the product needed to be granulated may be sensitive to moisture and heat. Dry granulation can be conducted on a press using slugging tooling or on a roller compactor commonly referred to as a chilsonator. Dry granulation equipment offers a wide range of pressure and roll types to attain proper densification. However the process may require repeated compaction steps to attain the proper granule end point. Process times are often reduced and equipment requirements are streamlined; therefore the cost is reduced. However, dry granulation often produces a higher percentage of fines or noncompacted products, which could compromise the quality or create yield problems for the tablet. It requires drugs or excipients with cohesive properties.
Fluidized bed granulationIt is a multiple step process performed in the same vessel to pr-heat, granulate and dry the powders. It is today a commonly used method in pharmaceuticals because it allows the individual company to more fully control the powder preparation process. It requires only one piece of machinery that mixes all the powders and granules on a bed of air. Tablet coatingThis is the last stage in tablet formulation and it is done to protect the tablet from temperature and humidity constraints. It is also done to mask the taste, give it special characteristics, distinction to the product, and prevent inadvertent contact with the drug substance. The most common forms of tablet coating are sugar coating and film coating. Coating is also done for because of these reason:
Sugar coating is done by rolling the tablets in heavy syrup, in a similar process to candy making. It is done to give tablets an attractive appearance and to make pill-taking less unpleasant. However the process is tedious and time-consuming and it requires the expertise of highly skilled technician. It also adds a substantial amount of weight to the tablet which can create some problems in packaging and distribution. In comparison to sugar coating, film coating is more durable, less bulky, and less time consuming. But it creates more difficulty in hiding tablet appearance. The purpose of this coating is to prevent dissolution of the tablet in the stomach, where the stomach acid may degrade the active ingredient, or where the time of passage may compromise its effectiveness, in favor of dissolution in the small intestine, where the active principle is better absorbed. Tablet pressesTablet presses, the machines that make the tablets, range from small, inexpensive bench-top models that make one tablet at a time, no more than a few thousand an hour. It takes only around a half-ton pressure, to large, computerized, industrial models that can make hundreds of thousands of tablets an hour with much greater pressure. Some tablet presses can make extremely large tablets, such as some of the toilet cleaning and deodorizing products or dishwasher soap. Mathematical anaylsisFriability is an important factor in tablet formulation to ensure that the tablet can stay intact and withhold its form from any outside force of pressure. Wo is the original weight of the tablets, and Wf is the final weight of the tablets after the collection is put through the friabilator.
Usually < 0.8% friability is considered satisfactory. References
Categories: Chemical engineering | Pharmaceutical industry |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Tablet_and_dose_preparation,_pharmaceutical_preparation". A list of authors is available in Wikipedia. |