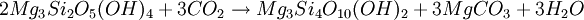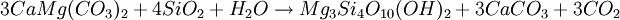To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Talc
Talc (derived from the Persian via Arabic talq) is a mineral composed of hydrated magnesium silicate with the chemical formula H2Mg3(SiO3)4 or Mg3Si4O10(OH)2. In loose form, it is the widely used substance known as talcum powder. It occurs as foliated to fibrous masses, its monoclinic crystals being so rare as to be almost unknown. It has a perfect basal cleavage, and the folia are non-elastic, although slightly flexible. It is sectile and very soft, with a hardness of 1 (Talc is the softest of the Mohs' scale of mineral hardness). It has a specific gravity of 2.5–2.8, a clear or dusty luster, and is translucent to opaque. Its colour ranges from white to grey or green and it has a distinctly greasy feel. Its streak is white. Product highlight
FormationTalc is a metamorphic mineral resulting from the metamorphism of magnesian minerals such as pyroxene, amphibole, olivine and other similar minerals in the presence of carbon dioxide and water. This is known as talc carbonation or steatization and produces a suite of rocks known as talc carbonates. Talc is primarily formed via hydration and carbonation of serpentine, via the following reaction; Serpentine + Carbon Dioxide → Talc + Magnesite + Water Talc can also be formed via a reaction between dolomite and silica, which is typical of skarnification of dolomites via silica-flooding in contact metamorphic aureoles; Dolomite + Silica + Water → Talc + Calcite + Carbon Dioxide Talc can also be formed from magnesian chlorite and quartz in blueschist and eclogite metamorphism via the following metamorphic reaction: Chlorite + Quartz → Kyanite + Talc + H2O In this reaction, the ratio of talc and kyanite is dependent on aluminium content with more aluminous rocks favoring production of kyanite. This is typically associated with high-pressure, low-temperature minerals such as phengite, garnet, glaucophane within the lower blueschist facies. Such rocks are typically white, friable, and fibrous, and are known as whiteschist. Talc is a tri-octahedral layered mineral; its structure is similar to that of pyrophyllite, but with magnesium in the octahedral sites of the composite layers.[1] OccurrenceTalc is a very common metamorphic mineral in metamorphic belts which contain ultramafic rocks, such as soapstone (a high-talc rock), and within whiteschist and blueschist metamorphic terranes. Prime examples of whiteschists include the Franciscan Metamorphic Belt of the western United States, the western European Alps especially in Italy, certain areas of the Musgrave Block, and some collisional orogens such as the Himalayas. Talc carbonated ultramafics are typical of many areas of the Archaean cratons, notably the komatiite belts of the Yilgarn Craton in Western Australia. Talc-carbonate ultramafics are also known from the Lachlan Fold Belt, eastern Australia, from Brazil, the Guiana Shield, and from the ophiolite belts of Turkey, Oman and the Middle East. Notable economic talc occurrences include the Mount Seabrook talc mine, Western Australia, formed upon a polydeformed, layered ultramafic intrusion. UsesA coarse grayish-green high-talc rock is soapstone or steatite and has been used for stoves, sinks, electrical switchboards, etc. Talc finds use as a cosmetic (talcum powder), as a lubricant, and as a filler in paper manufacture. Talc is used in baby powder, an astringent powder used for preventing rashes on the area covered by a diaper (see diaper rash). Most tailor's chalk is talc, as is the chalk often used for welding or metalworking. Talc is also used as food additive or in pharmaceutical products. In medicine talc is used as a pleurodesis agent to prevent recurrent pneumothorax. In the European Community the additive number is E553b. Recently it has come into popularity as a base for cocaine for several reasons. The first, and most common though, is that its chemical properties interfere with the sense of smell in most dogs, causing the drug to be next to undetectable by drug dogs. SafetySeveral studies have established preliminary links between talc and pulmonary issues,[2] lung cancer,[3][4] skin cancer and ovarian cancer.[5] This is a major concern considering talc's widespread commercial and household use. In 1993, a US National Toxicology Program report found that cosmetic grade talc caused tumours in animals, even though it contained no asbestos-like fibres.[3] Scientists have been aware of the toxicity of talc since the late 1960s, and in 1971 researchers found particles of talc embedded in 75 percent of the ovarian tumors studied.[citation needed] However, the U.S. Food and Drug Administration (FDA) considers non-asbestiform talc, that is talc which does not contain potentially carcinogenic asbestiform amphibole fibers, to be Generally recognized as safe (GRAS) for use in cosmetics. [6] See also
References
Categories: Phyllosilicates | Magnesium minerals | Cosmetic chemicals |
|||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Talc". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||