To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Total inorganic carbonThe total inorganic carbon (CT, or TIC) or Dissolved inorganic carbon (DIC) is the sum of inorganic carbon species in a solution. The inorganic carbon species include carbon dioxide, carbonic acid, bicarbonate anion, and carbonate anion. It is customary to express carbon dioxide and carbonic acid simultaneously as CO2* . CT is an important parameter when making measurements related to the pH of natural aqueous systems, and carbon dioxide flux estimates.
Product highlightwhere,
Each of these species are intimately related by the following pH driven chemical equilibria:
Total inorganic carbon is measured by the acidification of the sample which drives the equilibria to CO2. This gas is then sparged from solution and trapped, and the quantity trapped is then measured, typically by infrared spectroscopy. See also
Categories: Chemical oceanography | Analytical chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Total_inorganic_carbon". A list of authors is available in Wikipedia. |





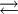 H2CO3
H2CO3 

