To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Ultraviolet photoelectron spectroscopyUltraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by ultraviolet photons, to determine molecular energy levels in the valence region. Product highlightIf Einstein’s photoelectric law is applied to a free molecule, the kinetic energy (EK) of an emitted photoelectron is given by
where h is Planck’s constant, ν is the frequency of the ionizing light, and I is an ionization energy corresponding to the energy of an occupied molecular orbital. Prior to 1960, virtually all measurements of photoelectron kinetic energies were for electrons emitted from metals and other solid surfaces. About 1956 Kai Siegbahn developed X-ray photoelectron spectroscopy (XPS) for surface chemical analysis. This method uses x-ray sources to study energy levels of atomic core electrons , and at the time had an energy resolution of about 1 eV (electronvolt).[1] The ultraviolet method (UPS) was developed to study the photoelectron spectra of free molecules in the gas phase by David W. Turner, a physical chemist at Oxford University, in a series of publications from 1962 to 1967.[2] As a photon source, he used a helium discharge lamp which emits a wavelength of 58.4 nm (corresponding to an energy of 21.2 eV) in the vacuum ultraviolet region. With this source Turner’s group obtained an energy resolution of 0.02 eV. Turner referred to the method as “molecular photoelectron spectroscopy”, now usually “Ultraviolet photoelectron spectroscopy” or UPS. As compared to XPS, UPS is limited to energy levels of valence electrons, but measures them more accurately. After 1967 commercial UPS spectrometers became available. [3] This method measures experimental molecular orbital energies for comparison with theoretical values from quantum chemistry, which was also extensively developed in the 1960s. The photoelectron spectrum of a molecule contains a series of peaks each corresponding to one valence-region molecular orbital energy level. Also, the high resolution allowed the observation of fine structure due to vibrational levels of the molecular ion, which facilitates the assignment of peaks to bonding, nonbonding or antibonding molecular orbitals. The method was later extended to the study of solid surfaces where it is usually described as photoemission spectroscopy. It is particularly sensitive to the surface region (to 10 nm depth), due to the short range of the emitted photoelectrons (compared to X-rays). It is therefore used to study adsorbed species and their binding to the surface, as well as their orientation on the surface. [4] References
Categories: Molecular physics | Spectroscopy | Surface chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Ultraviolet_photoelectron_spectroscopy". A list of authors is available in Wikipedia. |





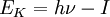 ,
,


