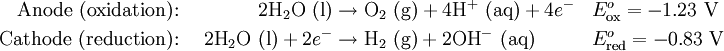To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electrolysis of waterElectrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water. This electrolytic process is used in some industrial applications when hydrogen is needed. An electrical power source is connected to two electrodes, or two plates, (typically made from some inert metal such as platinum or stainless steel) which are placed in the water. Hydrogen will appear at the cathode (the negatively charged electrode, where electrons are pumped into the water), and oxygen will appear at the anode (the positively charged electrode). The generated amount of hydrogen is twice the amount of oxygen, and both are proportional to the total electrical charge that was sent through the water. Electrolysis of pure water is very slow; it is sped up dramatically by adding an electrolyte (such as a salt, an acid or a base). Product highlight
EquationsIn the water at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas: At the positively charged anode, an oxidation reaction occurs, generating oxygen gas and giving electrons to the anode to complete the circuit: Combining these two reactions yields the overall decomposition of water into oxygen and hydrogen: The number of hydrogen molecules produced is thus twice the number of oxygen molecules. Assuming equal temperature and pressure for both gases, the produced hydrogen gas has therefore twice the volume of the produced oxygen gas. The number of electrons pushed through the water is twice the number of generated hydrogen molecules and four times the number of generated oxygen molecules. Spontaneity of the processDecomposition of pure water into hydrogen and oxygen at standard temperature and pressure is not favorable in thermodynamical terms, as half of the reaction's standard potential are negative values. On the other hand, Gibbs free energy for the process at standard conditions is a higher positive value, about 474.4 kJ. Those considerations makes the process "impossible" to occur without adding electrolytes in the solution with necessary energy supplied by an external electrical power source. Electrolyte selectionIf the above described processes occur in pure water, H+ cations will accumulate at the anode and OH− anions will accumulate at the cathode. This can be verified by adding a pH indicator to the water: the water near the anode is acidic while the water near the cathode is basic. These charged ions will repel the further flow of electricity until they have diffused away, a slow process. This is why pure water conducts electricity poorly and why electrolysis of pure water proceeds slowly. If a water-soluble electrolyte is added, the conductivity of the water rises considerably. The electrolyte disassociates into cations and anions; the anions rush towards the anode and neutralize the buildup of positively charged H+ there; similarly, the cations rush towards the cathode and neutralize the buildup of negatively charged OH− there. This allows the continued flow of electricity.[1] Care must be taken in choosing an electrolyte, since an anion from the electrolyte is in competition with the hydroxide ions to give up an electron. An electrolyte anion with less standard electrode potential than hydroxide will be oxidized instead of the hydroxide, and no oxygen gas will be produced. A cation with a greater standard electrode potential than a hydrogen ion will be reduced in its stead, and no hydrogen gas will be produced. The following cations have lower electrode potential than H+ and are therefore suitable for use as electrolyte cations: Li+, Rb+, K+, Cs+, Ba2+, Sr2+, Ca2+, Na+, and Mg2+. Sodium and lithium are frequently used, as they form inexpensive, soluble salts. If an acid is used as the electrolyte, the cation is H+, and there is no competitor for the H+ created by disassociating water. The most commonly used anion is sulfate (SO42-), as it is very difficult to oxidize, with the standard potential for oxidation of this ion to the peroxydisulfate ion being −0.22 volts. Strong acids such as Sulphuric acid (H2SO4), and strong bases such as potassium hydroxide (KOH), and sodium hydroxide (NaOH) are frequently used as electrolytes. TechniquesFundamental DemonstrationTwo leads, running from the terminals of a battery, are placed in a cup of water with a quantity of electrolyte added to establish conductivity. Hydrogen and Oxygen gases will stream from the oppositely charged electrode. Oxygen will collect at the anode and hydrogen will collect at the cathode.
Hofmann voltameterThe Hofmann voltameter is often used as a small-scale electrolytic cell. It consists of three joined upright cylinders. The inner cylinder is open at the top to allow the addition of water and the electrolyte. A platinum electrode is placed at the bottom of each of the two side cylinders, connected to the positive and negative terminals of a source of electricity. When current is run through the hofmann voltameter, gaseous oxygen forms at the anode and gaseous hydrogen at the cathode. Each gas displaces water and collects at the top of the two outer tubes, where it can be drawn off with a stopcock. Industrial electrolysisMany industrial electrolysis cells are very similar to Hofmann voltameters, with complex platinum plates or honeycombs as electrodes. Hydrogen gas is usually created and collected on site for use in other chemical processes, although in case of refineries it then makes more sense to produce it from natural gas. It can also be produced as a by-product, for example in brine electrolysis. High-temperature electrolysisHigh-temperature electrolysis (also HTE or steam electrolysis) is a method currently being investigated for water electrolysis with a heat engine. High temperature electrolysis is more efficient than traditional room-temperature electrolysis because some of the energy is supplied as heat, which is cheaper than electricity, and because the electrolysis reaction is more efficient at higher temperatures. ApplicationsAbout four percent of hydrogen gas produced worldwide is created by electrolysis, and normally used on site. Hydrogen is used for the creation of ammonia for fertilizer via the Haber process, and for converting heavy petroleum sources to lighter fractions via hydrocracking. There is some speculation about future development of hydrogen as an energy carrier in a hydrogen economy, although the rapid evolution of electric battery technology makes overall efficiency a major consideration. Hydrogen fuel injection is a concept having a long history; its popularity has reemerged in recent years. EfficiencyWater electrolysis does not convert 100% of the electrical energy into the chemical energy of hydrogen. The process loses energy because ions in the water need to move to carry electricity, and this movement ultimately heats up the water. The energy efficiency of water electrolysis varies widely. Some report 50–70%[1], while others report 80–94%.[2] These values refer only to the efficiency of converting electrical energy into hydrogen's chemical energy. The energy lost in generating the electricity is not included. For instance, when considering a power plant that converts the heat of nuclear reactions into hydrogen via electrolysis, the total efficiency may be closer to 25–45%.[3] See also
References
Categories: Electrolysis | Hydrogen production |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electrolysis_of_water". A list of authors is available in Wikipedia. |