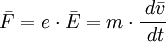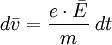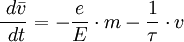To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Wiedemann-Franz lawIn physics, the Wiedemann-Franz law states that the ratio of the thermal conductivity (K) to the electrical conductivity (σ) of a metal is proportional to the temperature (T). Product highlightTheoretically, the proportionality constant L, known as the Lorenz number, is equal to
This empirical law is named after Gustav Wiedemann and Rudolf Franz, who in 1853 reported that K/σ has approximately the same value for different metals at the same temperature. The proportionality of K/σ with temperature was discovered by Ludvig Lorenz in 1872. Qualitatively, this relationship is based upon the fact that the heat and electrical transport both involve the free electrons in the metal. The thermal conductivity increases with the average particle velocity since this increases the forward transport of energy. The electrical conductivity, on the other hand, decreases while particle velocity increases because the collisions divert the electrons from forward transport of charge. The mathematical expression of the law can be derived as following. Electrical conduction of metals is a well known phenomenon and is attributed to the rather free conduction electrons. It is measured as sketched in the figure. The current density j is observed to be proportional to the applied electric field and follows Ohm's law where the prefactor is the specific conductivity. Since the electric field and the current density are vectors we have expressed Ohm's law here in bold face. The conductivity can in general be expressed as a tensor of the second rank (3x3 matrix). Here we restrict the discussion to isotropic, i.e. scalar conductivity. The specific resistivity is the inverse of the conductivity. Both parameters will be used in the following. Drude (around the year 1900) realized that the phenomenological description of conductivity can be formulated quite generally (electron-, ion-, heat- etc. conductivity). Although the phenomenological description is incorrect for conduction electrons, it can serve as a preliminary treatment. The assumption is that the electrons move freely in the solid like in an ideal gas. The force applied to the electron by the electric field leads to an acceleration according to This would lead, however, to an infinite velocity. The further assumption therefore is that the electrons bump into obstacles (like defects or phonons) once in a while which limits their free flight. This establishes an average or drift velocity Vd. The drift velocity is related to the average scattering time as becomes evident from the following relations. See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Wiedemann-Franz_law". A list of authors is available in Wikipedia. |


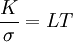



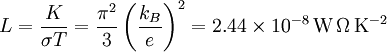 .
.