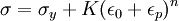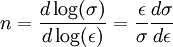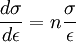To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Work hardeningWork hardening, strain hardening, or cold work is the strengthening of a material by increasing the material's dislocation density. In metallic crystals, irreversible deformation is usually carried out on a microscopic scale by defects called dislocations,which are created by fluctuations in local stress fields within the material culminating in a lattice rearrangement as the dislocations propagate through the lattice. At normal temperatures the dislocations are not annihilated by annealing. Instead, the dislocations accumulate, interact with one another, and serve as pinning points or obstacles that significantly impede their motion. This leads to an increase in the yield strength of the material and a subsequent decrease in ductility. Any material with a reasonably high melting point such as metals and alloys can be strengthened in this fashion. Alloys not amenable to heat treatment, including low-carbon steel, are often work-hardened. Some materials cannot be work-hardened at normal ambient temperatures; for example indium, which has a low melting point. This makes indium suitable for manufacturing gaskets, which deform to fill gaps, for high-vacuum use. Work hardening is often produced by the same process that shapes the metal into its final form, including cold rolling (contrast hot rolling) and cold drawing. Techniques have been designed to maintain the general shape of the workpiece during work hardening, including shot peening and constant channel angular pressing. A material's work hardenability can be predicted by analyzing a stress-strain curve, or studied in context by performing hardness tests before and after a process. Cold forming is a type of cold working that involves forging operations, such as extrusion, drawing or coining, performed at low temperatures. Cold working may also refer to the process through which a material is given this quality. Such deformation increases the concentration of dislocations which may subsequently form low-angle grain boundaries surrounding sub-grains. Cold working generally results in a higher yield strength as a result of the increased number of dislocations and the Hall-Petch effect of the sub-grains, and a decrease in ductility. The effects of cold working may be reversed by annealing the material at high temperatures where recovery and recrystallization reduce the dislocation density. Product highlight
TheoryElastic and plastic deformationMain article: Deformation Work hardening is a consequence of plastic deformation, or a permanent deformity to a material. This is distinct from elastic deformation, which is a reversible deformity. Most materials do not exhibit one or the other, but rather a combination of the two. The following discussion mostly applies to metals, especially steels, which are well studied. Work hardening occurs most notably for ductile materials such as metals. Ductility is the ability of a material to undergo large plastic deformations before fracture (for example, bending a steel rod until it finally breaks). The tensile test is widely used to study deformation mechanisms. This is because under compression, most materials will experience trivial (lattice mismatch) and non-trivial (buckling) events before plastic deformation or fracture occur. Hence the intermediate processes that occur to the material under uniaxial compression before the incidence of plastic deformation make the compressive test fraught with difficulties. A material generally deforms elastically if it is under the influence of small forces, allowing the material to readily return to its original shape when the deforming force is removed. This behavior in materials is governed by Hooke's Law. Materials behave elastically until the deforming force increases beyond the elastic limit, also known as the yield stress. At this point, the material is rendered permanently deformed and fails to return to its original shape when the force is removed. This phenomenon is called plastic deformation. For example, if one stretches a coil spring up to a certain point, it will return to its original shape, but once it is stretched beyond the elastic limit, it will remain deformed and won't return to its original state. Elastic deformation stretches atomic bonds in the material away from their equilibrium radius of separation of a bond, without applying enough energy to break the inter-atomic bonds. Plastic deformation, on the other hand, breaks interatomic bonds, and involves the rearrangement of atoms in a solid material. Dislocations and lattice strain fieldsMain article: Dislocation In materials science parlance, dislocations are defined as line defects in a material's crystallographic structure. They are surrounded by relatively strained(and weaker) bonds than the bonds between the constituents of the regular crystal lattice. This explains why these bonds break first during plastic deformation. Like any thermodynamic system, the crystals tend to lower their energy through bond formation between constituents of the crystal. Thus the dislocations interact with one another and the atoms of the crystal. The results in a lower but energetically favorable energy conformation of the crystal. Dislocations are a "negative-entity" in that they do not exist: they are merely vacancies in the host medium which does exist. As such, the material itself does not move much. To a much greater extent visible "motion" is movement in a bonding pattern of largely stationary atoms. (Please see for further discussion: edge dislocation, screw dislocation) The strained bonds around a dislocation are characterized by lattice strain fields. For example, there are compressively strained bonds directly next to an edge dislocation and tensilely strained bonds beyond the end of an edge dislocation. These form compressive strain fields and tensile strain fields, respectively. Strain fields are analogous to electric fields in certain ways. Additionally, the strain fields of dislocations, obey the laws of attraction and repulsion. The visible (macroscopic) results of plastic deformation are the result of microscopic dislocation motion. For example, the stretching of a steel rod in a tensile tester is accommodated through dislocation motion on the atomic scale. Increase of dislocations and work hardeningIncrease in the number of dislocations is a quantification of work hardening. Plastic deformation occurs as a consequence of work being done on a material; energy is added to the material. In addition, the energy is almost always applied fast enough and in large enough magnitude to not only move existing dislocations, but also to produce a great number of new dislocations by jarring or working the material sufficiently enough. Yield strength is increased in a cold-worked material. Using lattice strain fields, it can be shown that an environment filled with dislocations will hinder the movement of any one dislocation. Because dislocation motion is hindered, plastic deformation cannot occur at normal stresses. Upon application of stresses just beyond the yield strength of the non-cold-worked material, a cold-worked material will continue to deform using the only mechanism available: elastic deformation. The regular scheme of stretching or compressing of electrical bonds (without dislocation motion) continues to occur, and the modulus of elasticity is unchanged. Eventually the stress is great enough to overcome the strain-field interactions and plastic deformation resumes. However, ductility of a work-hardened material is decreased. Ductility is the extent to which a material can undergo plastic deformation, that is, it is how far a material can be plastically deformed before fracture. A cold-worked material is, in effect, a normal material that has already been extended through part of its allowed plastic deformation. If dislocation motion and plastic deformation have been hindered enough by dislocation accumulation, and stretching of electronic bonds and elastic deformation have reached their limit, a third mode of deformation occurs: fracture. Governing EquationsThe stress, τ, of dislocation is dependent on the shear modulus, G, the lattice constant, b, and the dislocation density, where τ0 is the intrinsic strength of the material with low dislocation density and α is a correction factor specific to the material. As shown in Figure 1 and the equation above, work hardening has a half root dependency on the number of dislocations. The material exhibits high strength if there are either high levels of dislocations (greater than 1014 dislocations per m2) or no dislocations. A moderate number of dislocations (between 10-7 and 10-9 dislocations per m2) typically results in low strength. ExampleFor an extreme example, in a tensile test a bar of steel is strained to just before the distance at which it usually fractures. The load is released smoothly and the material relieves some of its strain by decreasing in length. The decrease in length is called the elastic recovery, and the end result is a work-hardened steel bar. The fraction of length recovered (length recovered/original length) is equal to the yield-stress divided by the modulus of elasticity. (Here we discuss true stress in order to account for the drastic decrease in diameter in this tensile test.) The length recovered after removing a load from a material just before it breaks is equal to the length recovered after removing a load just before it enters plastic deformation. The work-hardened steel bar has a large enough number of dislocations that the strain field interaction prevents all plastic deformation. Subsequent deformation requires a stress that varies linearly with the strain observed, the slope of the graph of stress vs. strain is the modulus of elasticity, as usual. The work-hardened steel bar fractures when the applied stress exceeds the usual fracture stress and the strain exceeds usual fracture strain. This may be considered to be the elastic limit and the yield stress is now equal to the fracture stress, which is of course, much higher than a non-work-hardened-steel yield stress. The amount of plastic deformation possible is zero, which is obviously less than the amount of plastic deformation possible for a non-work-hardened material. Thus, the ductility of the cold-worked bar is drastically reduced. Substantial and prolonged cavitation can also produce strain hardening. Mathematical descriptionsThere are two common mathematical descriptions of the work hardening phenomenon. Hollomon's equation is a power law relationship between the stress and the amount of plastic strain εp. Ludwik's equation is similar but includes the yield stress σy
where K is the strength index and n is the strain hardening index.
This equation can be evaluated from the slope of a log(σ) - log(ε) plot. Rearranging allows a determination of the rate of strain hardening at a given stress and strain
See also
Categories: Materials science | Metals processes |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Work_hardening". A list of authors is available in Wikipedia. |





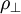 :
:
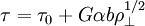
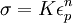 (Hollomon's)
(Hollomon's)
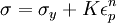 (Ludwik's)
(Ludwik's)