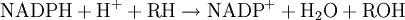To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Xenobiotic metabolismXenobiotic metabolism is the set of metabolic pathways that chemically modify xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as drugs and poisons. These pathways are a form of biotransformation present in all major groups of organisms, and are probably of ancient origin. These reactions often act to detoxify poisonous compounds, although in some cases the intermediates in xenobiotic metabolism can themselves be the cause of toxic effects. Xenobiotic metabolism is commonly divided into three phases. In phase I, enzymes such as cytochrome P450 oxidases introduce reactive or polar groups into xenobiotics. These modified compounds are then conjugated to polar compounds in phase II reactions. These reactions are catalysed by transferase enzymes such as glutathione S-transferases. Finally, in phase III, the conjugated xenobiotics may be further processed, before being recognised by efflux transporters and pumped out of cells. The reactions in these pathways are of particular interest in medicine as part of drug metabolism and as a factor contributing to multidrug resistance in infectious diseases and cancer chemotherapy. The actions of some drugs as substrates or inhibitors of enzymes involved in xenobiotic metabolism are a common reason for hazardous drug interactions. These pathways are also important in environmental science, with the xenobiotic metabolism of microorganisms determining whether a pollutant will be broken down during bioremediation, or persist in the environment. Product highlight
Permeability barriers and detoxificationA major characteristic of xenobiotic toxic stress is that which exact compounds an organism is exposed to will be largely unpredictable, and may differ widely over time.[1] The major challenge faced by xenobiotic detoxification systems is that they must therefore be able to remove the almost limitless number of possible xenobiotic compounds from the complex mixture of chemicals involved in normal metabolism. The solution that has evolved to this problem is an elegant combination of physical barriers and low-specificity enzymatic systems. All organisms use cell membranes as hydrophobic permeability barriers to control access to their internal environment. Polar compounds cannot diffuse across these cell membranes and the uptake of useful molecules is mediated through transport proteins that specifically select substrates from the extracellular mixture. This selective uptake means that most hydrophilic molecules cannot enter cells, since they are not recognised by any specific transporters.[2] In contrast, the diffusion of hydrophobic compounds across these barriers cannot be controlled and organisms therefore cannot exclude lipid-soluble xenobiotics using membrane barriers. However, the existence of a permeability barrier means that organisms were able to evolve detoxification systems that exploit the hydrophobicity common to membrane-permeable xenobiotics. These systems therefore solve the specificity problem by possessing such broad substrate specificities that they metabolise almost any non-polar compound.[1] Useful metabolites are excluded since they are polar, and in general contain one or more charged groups. The detoxification of the reactive by-products of normal metabolism cannot achieved by the systems outlined above. This is since these species are derived from normal cellular constituents and usually share their polar characteristics. However, since these compounds are few in number, specific enzymes can recognize and remove them. Examples of these specific detoxification systems are the glyoxalase system, which removes the reactive aldehyde methylglyoxal,[3] and the various antioxidant systems that eliminate reactive oxygen species.[4] Phases of detoxificationThe metabolism of xenobiotics is often divided into three phases: modification, conjugation and excretion. These reactions act in concert to detoxify xenobiotics and remove them from cells. Phase I - modificationIn phase I a variety of enzymes act to introduce reactive and polar groups into their substrates. One of the most common modifications is hydroxylation catalysed by the cytochrome P-450-dependent mixed-function oxidase system. These enzyme complexes act to incorporate an atom of oxygen into nonactivated hydrocarbons, which can result in either the introduction of hydroxyl groups or N-, O- and S-dealkylation of substrates.[5] The reaction mechanism of the P-450 oxidases proceeds through the reduction of cytochrome-bound oxygen and the generation of a highly reactive oxyferryl species, according to the following scheme:[6] Phase II - conjugationIn subsequent phase II reactions, these activated xenobiotic metabolites are conjugated with charged species such as glutathione (GSH), sulphate, glycine or glucuronic acid. These reactions are catalysed by a large group of broad-specificity transferases, which in combination can metabolise almost any hydrophobic compound which contains nucleophilic or electrophilic groups.[1] One of the most important of these groups are the glutathione S-transferases (GSTs). The addition of large anionic groups (such as GSH) detoxifies reactive electrophiles and produces more polar metabolites that cannot diffuse across membranes and may therefore be actively transported. Phase III - further modification and excretionAfter phase II reactions, the xenobiotic conjugates may be further metabolised. A common example is the processing of glutathione conjugates to acetylcysteine (mercapturic acid) conjugates.[7] Here, the γ-glutamate and glycine residues in the glutathione molecule are removed by Gamma glutamyl transpeptidase and dipeptidases. Finally, the cystine residue in the conjugate is acetylated. Conjugates and their metabolites can be excreted from cells in phase III of their metabolism, with the anionic groups acting as affinity tags for a variety of membrane transporters of the multidrug resistance protein (MRP) family.[8] These proteins are members of the family of ATP-binding cassette transporters and can catalyse the ATP-dependent transport of a huge variety of hydrophobic anions,[9] and thus act to remove phase II products to the extracellular medium, where they may be further metabolised or excreted.[10] Endogenous toxinsThe detoxification of endogenous reactive metabolites such as peroxides and reactive aldehydes often cannot be achieved by the system described above. This is the result of these species being derived from normal cellular constituents and usually sharing their polar characteristics. However, since these compounds are few in number, it is possible for enzymatic systems to utilize specific molecular recognition to recognize and remove them. The similarity of these molecules to useful metabolites therefore means that different detoxification enzymes are usually required for the metabolism of each group of endogenous toxins. Examples of these specific detoxification systems are the glyoxalase system, which acts to dispose of the reactive aldehyde methylglyoxal, and the various antioxidant systems that remove reactive oxygen species. HistoryStudies on how people transform the substances that they ingest began in the mid-nineteenth century, with chemists discovering that organic chemicals such as benzaldehyde could be oxidized and conjugated to amino acids in the human body.[11] During the remainder of the nineteenth century, several other basic detoxification reactions were discovered, such as methylation, acetylation and sulfonation. In the early twentieth century, work moved on to the investigation of the enzymes and pathways that were responsible for the production of these metabolites. This field became defined as a separate area of study with the publication by Richard Williams of the book Detoxication mechanisms in 1947.[12] This modern biochemical research resulted in the identification of glutathione S-transferases in 1961,[13] followed by the discovery of cytochrome P450s in 1962,[14] and the realization of their central role in xenobiotic metabolism in 1963.[15][16] See alsoReferences
Further reading
Drug metabolism
Microbial biodegradation
History
|
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Xenobiotic_metabolism". A list of authors is available in Wikipedia. |