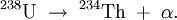To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (two protons and two neutrons bound together into a particle identical to a helium nucleus) and transforms (or 'decays') into an atom with a mass number 4 less and atomic number 2 less. For example: although this is typically written as: (The second form is preferred because the first form appears electrically unbalanced. Fundamentally, the recoiling nucleus is very quickly stripped of two electrons to neutralize the ionized helium cation.) An alpha particle is identical to a helium-4 nucleus, and both mass number and atomic number are the same. Alpha decay is a form of nuclear fission where the parent atom splits into two daughter products. Alpha decay is fundamentally a quantum tunneling process. Unlike beta decay, alpha decay is governed by the strong nuclear force. Alpha particles have a typical kinetic energy of 5 MeV (that is ≈0.13% of their total energy, i.e. 110 TJ/kg) and a speed of 15,000 km/s. This corresponds to a speed of around 0.05c. Because of their relatively large mass, +2 charge and relatively low velocity, they are very likely to interact with other atoms and lose their energy, so they are effectively absorbed within a few centimeters of air. Most of the helium produced on Earth comes from the alpha decay of underground deposits of minerals containing uranium or thorium. The helium is brought to the surface as a by-product of natural gas production. Product highlight
HistoryBy 1928, George Gamow had solved the theory of the alpha decay via tunneling. The alpha particle is trapped in a potential well by the nucleus. Classically, it is forbidden to escape, but according to the then newly discovered principles of Quantum mechanics, it has a tiny (but non-zero) probability of "tunneling" through the barrier and appearing on the other side to escape the nucleus. UsesAmericium-241 is used in smoke detectors. The alpha particles ionize air between a small gap, leading to a small current that can be easily interrupted by smoke particles. Alpha decay can provide a safe power source for radioisotope thermoelectric generators used for space probes and artificial heart pacemakers. Alpha decay is much more easily shielded against than other forms of radioactive decay. Plutonium-238, for example, requires only 2.5 mm of lead shielding to protect against unwanted radiation. ToxicityBeing relatively heavy and positively charged, alpha particles tend to have a very short mean free path, and quickly lose kinetic energy within a short distance of their source. This results in several MeV being deposited in a relatively small area. This increases the chance of cellular damage in cases of internal contamination. In general, external alpha radiation is not harmful since alpha particles are effectively shielded by a few centimeters of air or the thin layer of dead skin cells. Even touching an alpha source is usually not harmful. If substances emitting alpha particles are ingested, inhaled, injected or introduced through the skin it could result in a measurable dose. The largest natural contributor to public radiation dose is radon, a naturally occurring, radioactive gas found in soil and rock[2]. If the gas is inhaled, some of the radon particles may attach to the inner lining of the lung. These particles continue to decay, emitting alpha particles which can damage cells in the lung tissue.[3]. The death of Marie Curie at age 66 from leukemia was likely caused by prolonged exposure to high doses of ionizing radiation. Curie worked extensively with Radium, which decays into Radon[4], along with other radioactive materials that emit beta and gamma rays. The 2006 assassination of Russian dissident Alexander Litvinenko is thought to have been caused by poisoning with Polonium-210, an alpha emitter. References
|
|||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Alpha_decay". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||