To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Isosbestic pointIn spectroscopy, an isosbestic point is a specific wavelength at which two (or more) chemical species have the same absorptivity. Such a point corresponds to a wavelength on an isosbestic plot at which the absorption spectra of two species cross each other; if both species are in the same concentration, then this represents the molar absorptivities (ε). This is equivalent to saying an isosbestic point is a wavelength where two or more species have equal extinction coefficients. Additional recommended knowledgeA pair of substances can have several isosbestic points in their spectra. When a 1-to-1 (one mole of reactant gives one mole of product) chemical reaction (including equilibria) involves a pair of substances with an isosbestic point, the absorbance of the reaction mixture at this wavelength remains invariant, regardless of the extent of reaction (or the position of the chemical equilibrium). This occurs because the two substances absorb light of that specific wavelength to the same extent, and the analytical concentration remains constant. For the reaction: the analytical concentration is the same at any point in the reaction:
The absorbance of the reaction mixture (assuming it depends only on X and Y) is:
But at the isosbestic point both molar absorptivities are the same:
Hence, the absorbance does not depend on the extent of reaction (i.e. in the particular concentrations of X and Y) ApplicationsIn chemical kinetics, isosbestic points are used as reference points in the study of reaction rates, as the absorbance at those wavelengths remains constant throughout the whole reaction. Isobestic points are used in medicine in a laboratory technique called oxymetry to determine hemoglobin concentration, regardless of its saturation. Oxyhaemoglobin and deoxyhaemoglobin have isosbestic points at 590 nm and near 800 nm. Isosbestic points are also used in clinical chemistry, as a quality assurance method, to verify the accuracy in the wavelength of a spectrophotometer. This is done by measuring the spectra of a standard substance at two different pH conditions (above and below the pKa of the susbtance). The standards used include potassium dichromate (isosbestic points at 339 and 445 nm), bromothymol blue (325 and 498 nm) and congo red (541 nm). The wavelength of the isosbestic point determined does not depend on the concentration of the substance used, and so, it becomes a very reliable reference. |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Isosbestic_point". A list of authors is available in Wikipedia. |





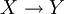
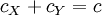 .
.
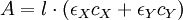 .
.
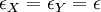 .
.