To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Metal nitrosyl
Metal nitrosyls are complexes that contain transition metals bonded to nitric oxide, NO.[1] Metal nitrosyls are important biologically. This large class of molecules continues to be of interest to researchers and technologists. Additional recommended knowledge
18-electron rule perspective
In the language of the 18-electron rule, NO is a three-electron ligand when the M-N-O angle is close to 180°. This bonding mode, "linear nitrosyl", is common. In some nitrosyl complexes, the M-N-O angle deviates strongly from linearity, approaching often 130°. Such bent species are related to classical organic and main group nitrosyl compounds, e.g. nitrosobenzene and nitrosyl chloride. In "bent NO" complexes, NO is considered to be a one-electron, pseudohalide-like ligand. Because of the two different bonding modes, metal nitrosyls are considered to be a thorny topic for those studying structural inorganic chemistry. Most transition metal nitrosyls are linear, most main group nitrosyls are bent. ExamplesTwo of the earliest nitrosyl complexes are "Roussin's Red Salt" and "Roussin's Black Salt". The Red Salt has the formula Na2[Fe2(NO)4S2]. The anion, [Fe2(NO)4S2]2−, can be viewed as an edge-shared bitetrahedron. Each Fe is bonded to two "linear" NO ligands and shares a pair of sulfido ligands with the other iron. The Black Salt is a more complex cluster with the formula Na[Fe4(NO)7S3]. The anion in this species has C3v point group symmetry. The Fe4S3 core is that of an incomplete cubane. The parent cubane is (FeNO)4S4. Simpler nitrosyl complexes include Co(NO)(CO)3 and Mn(NO)(CO)4, which are structurally related to Ni(CO)4 and Fe(CO)5. A medicinally important nitrosyl is the nitroprusside anion, [Fe(CN)5NO]2−. The signalling function of NO is effected via its complexation to haeme proteins. PreparationMost metal nitrosyls are prepared by treatment of a metal with NO gas. Others have been prepared by oxidation of hydroxylamine complexes. A third method involves treatment of metal nitrito complexes with protic acids. ReactionsSince nitrogen is more electronegative than carbon, metal-nitrosyl complexes tend to be more electrophilic than related metal carbonyl complexes. Nucleophiles often add to the nitrogen.[2] The NO ligand also exhibits many reactions. The most important is the acid/base equilibrium:
This reaction illustrates the fact that linear nitrosyls can be viewed an acid anhydride. In rare but noteworthy cases, NO is cleaved by metal centers:
The nitrogen atom in bent metal nitrosyls is basic, thus can be oxidized, alkylated, and protonated, e.g.:
References
Categories: Oxides | Nitrogen compounds | Organometallic chemistry |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Metal_nitrosyl". A list of authors is available in Wikipedia. |





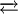 LnMNO2 + H+
LnMNO2 + H+


