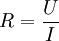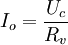To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
PotentiostatA potentiostat is a control and measuring device that, in an electrolytic cell, keeps the potential of the working electrode at a constant level respect to the reference electrode. It consists of an electric circuit which controls the potential across the cell by sensing changes in its resistance, varying accordingly the current supplied to the system: a higher resistance will result in a decreased current, while a lower resistance will result in an increased current, in order to keep the voltage constant. It is a simple application of Ohm's law Additional recommended knowledgeAs a result, the variable system resistance and the controlled current are inversely proportional
In a potentiostatic coulometry experiment, the cathode is usually a platinum electrode with a large surface area or a mercury pool, although other particularly reactive electrodes can be used in rare circumstances. The functionality of a potentiostat can be extended by virtual instrumentation: in virtual instruments the potentiostat is used as an actuator. See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Potentiostat". A list of authors is available in Wikipedia. |