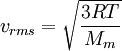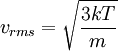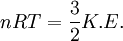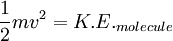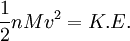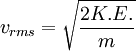To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Root mean square speedRoot mean square speed is the measure of the speed of particles in a gas that is most convenient for problem solving within the kinetic theory of gases. It is given by the formula Additional recommended knowledgewhere vrms is the root mean square speed, Mm is the molar mass of the gas, R is the Molar gas constant, and T is the temperature in Kelvin. This works well for both ideal gases like helium and for molecular gases like diatomic oxygen. This is because despite the larger internal energy in many molecules (compared to that for an atom), 3RT/2 is still the mean translational kinetic energy. This can also be written in terms of the Boltzmann constant (k) as where m is the mass of the gas. This can be derived with energy methods: where K.E. is the kinetic energy. Given that v2 ignores direction, it is logical to assume that the formula can be extended to the entire sample, replacing m with the entire sample's mass, equal to the molar mass times the number of moles, "nM", yielding Therefore which is equivalent. See also |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Root_mean_square_speed". A list of authors is available in Wikipedia. |