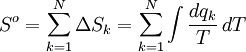To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Standard molar entropyIn chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (NOT STP). The standard molar entropy is usually given the symbol So, and the units J mol−1 K−1 (joules per mole kelvin). Unlike standard enthalpies of formation, the value of So is an absolute. That is, an element in its standard state has a nonzero value of So at room temperature. The entropy of an element can be 0 J mol−1 K−1 only at 0 K, according to the third law of thermodynamics. Additional recommended knowledge
ThermodynamicsIf a mole of substance were at 0 K, then warmed by its surroundings to 298 K, its total molar entropy would be the addition of all N individual contributions: Here, dqk/T represents a very small exchange of heat energy at temperature T. The total molar entropy is the sum of many small changes in molar entropy, where each small change can be considered a reversible process. ChemistryThe standard molar entropy of a gas at STP includes contributions from:[1]
Changes in entropy are associated with phase transitions and chemical reactions. Chemical equations make use of the standard molar entropy of reactants and products to find the standard entropy of reaction:
The standard entropy of reaction helps determine whether the reaction will take place spontaneously. According to the second law of thermodynamics, a spontaneous reaction always results in an increase in total entropy of the system and its surroundings:
See alsoReferencesCategories: Chemical properties | Thermodynamic entropy |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Standard_molar_entropy". A list of authors is available in Wikipedia. |