Salt boosts creation of 2-D materials
A dash of salt can simplify the creation of two-dimensional materials, and thanks to Rice University scientists, the reason is becoming clear.
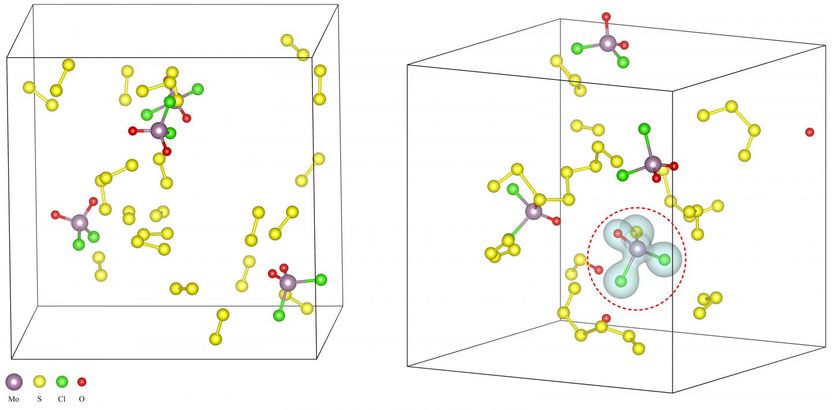
Rice University scientists built computer models of intermediate reactions to understand why salt lowers reaction temperatures in the synthesis of two-dimensional compounds. Above left, molybdenum oxychloride precursor molecules undergo sulfurization in which sulfur atoms replace oxygen atoms. That sets up the material to form new compounds. At right, the calculations show the charge densities of the new molecules.
Yakobson Group/Rice University
Boris Yakobson, a Rice professor of materials science and nanoengineering and of chemistry, was the go-to expert when a group of labs in Singapore, China, Japan and Taiwan used salt to make a "library" of 2-D materials that combined transition metals and chalcogens.
These compounds could lead to smaller and faster transistors, photovoltaics, sensors and catalysts, according to the researchers.
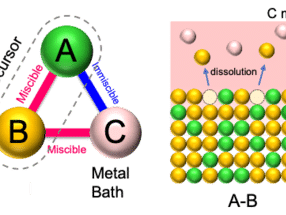
Through first-principle molecular dynamics simulations and accurate energy computations, Yakobson and his colleagues determined that salt reduces the temperature at which some elements interact in a chemical vapor deposition (CVD) furnace. That makes it easier to form atom-thick layers similar to graphene but with the potential to customize their chemical composition for specific layer-material and accordingly electrical, optical, catalytic and other useful properties.
The team led by Zheng Liu of Nanyang Technological University in Singapore used its seasoned technique with CVD to create 47 compounds of metal chalcogenides (which contain a chalcogen and an electropositive metal). Most of the new compounds had two ingredients, but some were alloys of three, four and even five. Many of the materials had been imagined and even coveted, Yakobson said, but never made.
In the CVD process, atoms excited by temperatures -- in this case between 600 and 850 degrees Celsius -- form a gas and ultimately settle on a substrate, linking to atoms of complementary chemistry to form monolayer crystals.
Researchers already suspected salt could facilitate the process, Yakobson said. Liu came to him to request a molecular model analysis to learn why salt made it easier to melt metals with chalcogens and get them to react. That would help them learn if it might work within the broader palette of the periodic table.
"They did impressively broad work to make a lot of new materials and to characterize each of them comprehensively," Yakobson said. "From our theoretical perspective, the novelty in this study is that we now have a better understanding of why adding plain salt lowers the melting point for these metal-oxides and especially reduces the energy barriers of the intermediates on the way to transforming them into chalcogenides."
Whether in the form of common table salt (sodium chloride) or more exotic compounds like potassium iodide, salt was found to allow chemical reactions by lowering the energetic barrier that otherwise prevents molecules from interacting at anything less than ultrahigh temperatures, Yakobson said.
"I call it a 'salt assault,'" he said. "This is important for synthesis. First, when you try to combine solid particles, no matter how small they are, they still have limited contact with each other. But if you melt them, with salt's help, you get a lot of contact on the molecular level.
"Second, salt reduces the sublimation point, where a solid undergoes a phase transformation to gas. It means more of the material's component molecules jump into the gas phase. That's good for general transport and contact issues and helps the reaction overall."
The Rice team discovered the process doesn't facilitate the formation of the 2-D-material itself directly so much as it allows for the formation of intermediate oxychlorides. These oxychlorides then lead to the 2-D chalcogenide growth.
Detailing this process required intensive atom-by-atom simulations, Yakobson said. These took weeks of heavy-duty computations of the quantum interactions among as few as about 100 atoms - all to show just 10 picoseconds of a reaction. "We only did four of the compounds because they were so computationally expensive, and the emerging picture was clear enough," Yakobson said.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.