Carbon's new champion
Rice U. theorists calculate atom-thick carbyne chains may be strongest material ever
Advertisement
carbyne will be the strongest of a new class of microscopic materials if and when anyone can make it in bulk. If they do, they'll find carbyne nanorods or nanoropes have a host of remarkable and useful properties, as described in a new paper by Rice University theoretical physicist Boris Yakobson and his group.
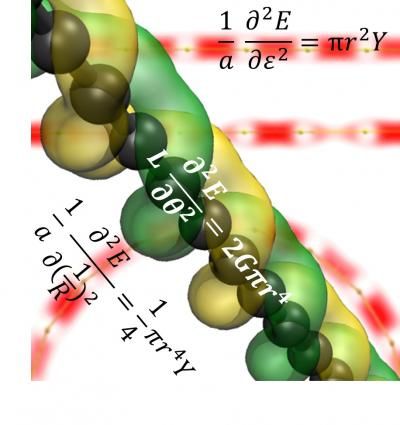
Carbyne
Vasilii Artyukhov/Rice University
Carbyne is a chain of carbon atoms held together by either double or alternating single and triple atomic bonds. That makes it a true one-dimensional material, unlike atom-thin sheets of graphene that have a top and a bottom or hollow nanotubes that have an inside and outside.
According to the portrait drawn from calculations by Yakobson and his group: Carbyne's tensile strength the ability to withstand stretching surpasses "that of any other known material" and is double that of graphene. (Scientists had already calculated it would take an elephant on a pencil to break through a sheet of graphene.)
It has twice the tensile stiffness of graphene and carbon nanotubes and nearly three times that of diamond. Stretching carbyne as little as 10 percent alters its electronic band gap significantly.
If outfitted with molecular handles at the ends, it can also be twisted to alter its band gap. With a 90-degree end-to-end rotation, it becomes a magnetic semiconductor.
Carbyne chains can take on side molecules that may make the chains suitable for energy storage. The material is stable at room temperature, largely resisting crosslinks with nearby chains.
That's a remarkable set of qualities for a simple string of carbon atoms, Yakobson said.
"You could look at it as an ultimately thin graphene ribbon, reduced to just one atom, or an ultimately thin nanotube," he said. It could be useful for nanomechanical systems, in spintronic devices, as sensors, as strong and light materials for mechanical applications or for energy storage.
Based on the calculations, he said carbyne might be the highest energy state for stable carbon. "People usually look for what is called the 'ground state,' the lowest possible energy configuration for atoms," Yakobson said. "For carbon, that would be graphite, followed by diamond, then nanotubes, then fullerenes. But nobody asks about the highest energy configuration. We think this may be it, a stable structure at the highest energy possible."