Electrochemical breakthrough turns nitrate waste into ammonia fuel
Copper-palladium hydride interfaces enable highly efficient electrochemical ammonia synthesis
Advertisement
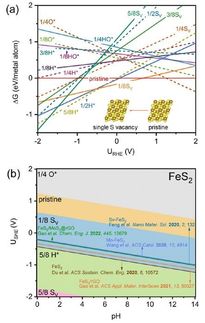
ammonia (NH3) is essential for agriculture and plays an important role in next-generation carbon-free energy systems. Renewable NH3 synthesis is a supplementary or alternative to the traditional Haber-Bosch process. The electrochemical nitrate reduction reaction (NO3−RR) to NH3 offers a promising route for sustainable NH3 production and effective nitrogen recovery. However, slow reaction kinetics and the competing hydrogen evolution reaction (HER) hinder the efficiency.
Copper-palladium hydride interfaces enable highly efficient electrochemical ammonia synthesis
DICP
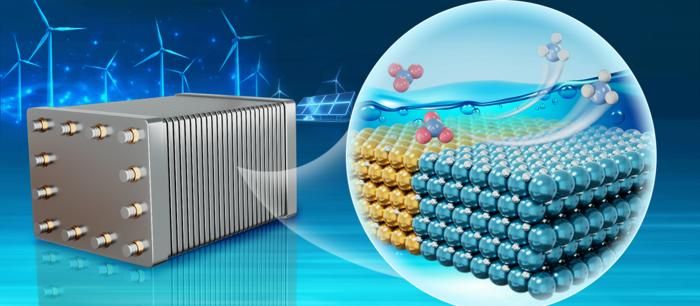
In a study published in Nature Synthesis, a team led by Prof. BAO Xinhe from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences developed a copper-palladium (CuPd) bimetallic catalyst, which dynamically formed abundant Cu-PdHx interfacial sites with high intrinsic catalytic activity in situ under NO3−RR conditions.
Under NO3−RR operating conditions, the CuPd bimetallic catalyst exhibited high intrinsic catalytic activity, achieving an NH3 production rate of 19.9 mmol h−1 cm−2 with a current density of 5 A cm–2 at a full-cell voltage of 2.56 V in a membrane electrode assembly (MEA) electrolyzer. It demonstrated good durability, maintaining a Faradaic efficiency of about 86.8% at 2 A cm−2 for over 1,000 hours.
Researchers revealed that the enhanced performance was attributed to the superior intrinsic activity of the Cu-PdHx interfaces. The hydrogen redistribution induced by the overflow at the Cu-PdHx interface modified the local electronic structure of the active sites, which optimized the NO3− adsorption, promoted the NH3 desorption, and provided a more energetically favorable reaction pathway for NH3 synthesis.
A scale-up demonstration using an electrolyzer stack with five 100 cm2 MEAs achieved an NH3 production rate of 8.7 mol h–1 at 500 A, and continuously produced 1.6 mol h–1 of NH3 at 100 A for 100 hours, underscoring the industrial applicability.
This study provides new insight into the structure-activity relationship of CuPd bimetallic sites. And it provides an effective strategy for enhancing intrinsic catalytic activity through the in situ construction of beneficial interfaces, enabling efficient conversion of nitrate pollutants into value-added NH3.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.