Towards an environmentally means of producing ammonia
Electrocatalytic ammonia synthesis
Advertisement
A team of researchers has unveiled a promising alternative to the conventional means of synthesizing ammonia, one that is more environmentally friendly. Details of their research were published in the Journal of Materials Chemistry A.
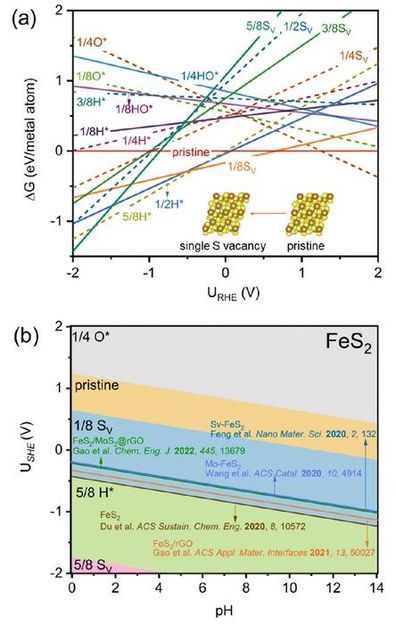
CALCULATED (A) 1D AND (B) 2D SURFACE POURBAIX DIAGRAMS OF FES2(111) CONSIDERING DIFFERENT COVERAGES OF SV, O*, H*, AND HO*. THE EXPERIMENTAL POTENTIALS AT THE HIGHEST FARADAIC EFFICIENCIES OF REPORTED FES2-BASED CATALYSTS ARE PLOTTED FOR A DIRECT COMPARISON.
Hao Li et al.
When Fritz Haber and Carl Bosch invented a means to synthesize ammonia from nitrogen and hydrogen gas in the early 20th century, it enabled the production of the chemical at an industrial level. To this day, the Haber-Bosch synthesis remains the dominant means of producing ammonia.
Yet the method has some environmental drawbacks. It is energy and resource intensive, and producing hydrogen gas often involves natural gas, which releases carbon dioxide as a by-product.
The electrochemical nitrogen reduction reaction (ENRR), where nitrogen gas from the air can be converted into ammonia using an electrical current, is seen as a promising and sustainable alternative. Pursuing high-performance and cost-effective ENRR catalysts, however, is an open challenge for achieving commercial-scale ambient ammonia production.
"We explored the potential of less-precious transition metal disulfides (TMS2) as catalysts for ENRR," says Hao Li, associate professor at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper. "Through meticulous analysis of electrochemistry-induced surface states, we uncovered a previously unrecognized factor contributing to their high ENRR performance: S-vacancy generation."
Li and his colleagues started with a typical ENRR TMS2 catalyst, iron disulfide (FeS2), where they observed that under ENRR conditions, S-vacancies can be easily generated on the catalyst surface. Through advanced computational simulations, they demonstrated that this electrochemistry-driven "in situ" generation of S-vacancies significantly enhances ENRR activity by promoting stronger N-N adsorption and activation.
Experimental observations confirmed their findings, which were also consistent with recent literature on ENRR potential windows reaching maximum Faradaic efficiency - the measure of the effectiveness of an electrochemical process in converting electrical energy into chemical energy or vice versa.
Their analysis also extended to other TMS2 catalysts (SnS2, MoS2, NiS2, and VS2), revealing a universal phenomenon of "in situ'' S-vacancy generation under ENRR potentials.
"Our research underscores the critical importance of considering surface states in the design of ENRR catalysts," adds Li. "By shedding light on the role of S-vacancies, we have provided a valuable roadmap for enhancing ENRR performance and accelerating the transition towards sustainable ammonia production."
This work was supported by AIMR Fusion Research and also received substantial support for the JSPS Postdoctoral Fellowship for Dr. Tianyi Wang in the Hao Li Lab.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.