New coating surface for superior rust resistance with 'colorless' color
Electrophoretic deposition coats metal with non-ionic polymer
Advertisement
Polymer chemists at Nagoya Institute of Technology in Japan invented a novel and simple coating process to color metals, leading to higher performance and saving the energy. The method involves a chemical modification to non-ionic polymers and nanotechnology.
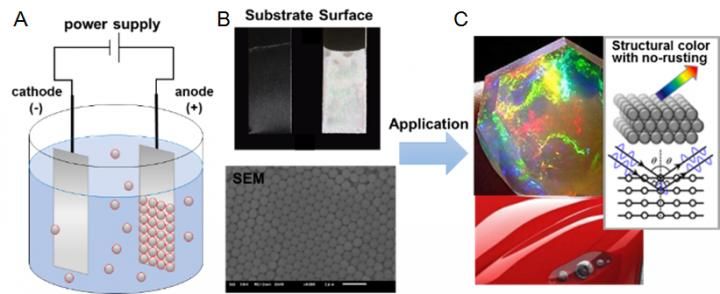
A) Illustration of the electrophoretic deposition (EPD) of sulfone-containing nano-latex shows negatively charged nano-particles in suspension migrating towards the positive electrode. B) The anode coated with aligned nano-particles (top: electrode, bottom: SEM image. Scale bar, 2000 nm). C) Images of application example and the light reflection by the colorless nano-particles.
NITECH
Electrophoretic deposition is a standard industrial method for coating material and is especially used for rust prevention. Current methods, however, require a complex and expensive process that requires three coating steps, adding cost and time. Professor Akinori Takasu and his team report novel non-ionic polymers that can be used with electrophoretic deposition, simplifying the coating to just one step and significantly reducing energy demands.
The key to the discovery was the addition of a specific chemical group to the non-ionic polymer molecule.
"It was accidentally found in a project designing a new material for dental implant. When a non-ionic polymer had a sulfonyl group, it moved towards the anode in electrophoresis" explains Takasu.
Previously, the research team showed that the resulting coating becomes incredibly thick when the electrophoretic disposition is applied at low voltages. Combining a set of findings made it possible to skip multiple coating processes on a metal for rust resistance. However, for commercial purposes, it is important that the coat come in any desired color. Takasu and his colleagues therefore looked at how the color properties of non-ionic polymers behaved in water after applied as coating.
He says, "Our breakthrough was to include this non-ionic polymer into nano-particles. The new particles show structural color like opal stones, a.k.a. colorless color. The wavenumber of the particle should be controllable by changing the size of the particles used to coat the surface," which determines the color emitted.
While Takasu could easily react the non-ionic polymers with the sulfonyl group, he found controlling for the size of the particles proved difficult. In this research, he and his team developed the size control technology and prepared the particles by soap-free emulsion copolymerization, which consistently gave nanoparticles 300 nm in size as an example. They then oxidized the particles in water to generate the sulfonyl group. Finally, electrophoretic deposition was applied to coat steel. Electron microscopic images confirmed that the particles uniformly covered the steel in a honeycomb pattern.
"I expect our study will lead to a new type of electrophoretic painting that can be applied to any coating technologies like cars and fibers," Takasu said. This technique overcomes problems such as color fading and damage from UV radiation because of structural coloring, thus will be provided for wider application of electrophoretic dispersion.