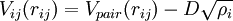To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Bond order potentialBond order potentials are a class of empirical (analytical) potentials used e.g. in molecular dynamics and molecular statics simulations. Examples include the Tersoff potential [1], the Brenner potential [2] , the Finnis-Sinclair potentials [3] and the second-moment tight-binding potentials. [4] They have the advantage over e.g. conventional molecular mechanics force fields that they can with the same parameters describe several different bonding states of an atom, and thus to some extent may be able to describe chemical reactions correctly. Product highlightThe potentials were developed partly independently of each other, but share the common idea that the strength of a chemical bond depends on the bonding environment (number of bonds and possibly also angles and distances) of an atom. This concept is based on the Linus Pauling bond order concept [5] [1] , and can be written in the form Vij(rij) = Vrepulsive(rij) + bijkVattractive(rij) This means that the potential is written as a simple pair potential depending on the distance between two atoms rij, but the strength of this bond is modified by the environment of the atom i via the bijk term. Alternatively, the energy can be written in the form
where ρi is an 'electron density' at the location of atom i. These two forms for the energy can be shown to be equivalent. [6] A more detailed summary of how the bond order concept can be motivated by the second-moment approximation of tight binding and both of these functional forms derived from it can be found in [7]
References
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Bond_order_potential". A list of authors is available in Wikipedia. |