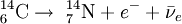To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Carbon-14
Carbon-14, 14C, or radiocarbon, is a radioactive isotope of carbon discovered on February 27, 1940, by Martin Kamen and Sam Ruben. Its nucleus contains 6 protons and 8 neutrons. Its presence in organic materials is used extensively as basis of the radiocarbon dating method to date archaeological, geological, and hydrogeological samples. There are three naturally occurring isotopes of carbon on Earth: 99% of the carbon is carbon-12, 1% is carbon-13, and carbon-14 occurs in trace amounts, making up as much as 1 part per trillion (0.0000000001%) of the carbon on the Earth. The half-life of carbon-14 is 5730±40 years. It decays into nitrogen-14 through beta-decay.[2] The activity of the modern radiocarbon standard[3]is about 14 disintegrations per minute (dpm) per gram carbon [4]. The atomic mass of carbon-14 is about 14.003241 amu. The different isotopes of carbon do not differ appreciably in their chemical properties. This is used in chemical research in a technique called carbon labeling: some carbon-12 atoms of a given compound are replaced with carbon-14 atoms (or some carbon-13 atoms) in order to trace them along chemical reactions involving the given compound. Product highlight
Origin and radioactive decay of carbon-14Carbon-14 is produced in the upper layers of the troposphere and the stratosphere by thermal neutrons absorbed by nitrogen atoms. When cosmic rays enter the atmosphere, they undergo various transformations, including the production of neutrons. The resulting neutrons (1n) participate in the following reaction:
The highest rate of carbon-14 production takes place at altitudes of 9 to 15 km (30,000 to 50,000 feet) and at high geomagnetic latitudes, but the carbon-14 readily mixes and becomes evenly distributed throughout the atmosphere and reacts with oxygen to form radioactive carbon dioxide. Carbon dioxide also dissolves in water and thus permeates the oceans. Carbon-14 can also be produced in ice by fast neutrons causing spallation reactions in oxygen. Carbon-14 then goes through radioactive beta decay. By emitting an electron and an anti-neutrino, carbon-14 (half life of 5730 years) decays into the stable, non-radioactive isotope nitrogen-14. Radiocarbon dating
Radiocarbon dating is a radiometric dating method that uses carbon-14 (14C) to determine the age of carbonaceous materials up to about 60,000 years old. The technique was discovered by Willard Libby and his colleagues in 1949[5] during his tenure as a professor at the University of Chicago. Libby estimated that the steady state radioactivity concentration of exchangeable carbon-14 would be about 14 disintegrations per minute (dpm) per gram. In 1960, he was awarded the Nobel Prize in chemistry for this work. One of the frequent uses of the technique is to date organic remains from archaeological sites. Plants fix atmospheric carbon during photosynthesis, so the level of C-14 in plants at the time wood is laid down, or in animals at the time they die, equals the level of C-14 in the atmosphere at that time. However, it decreases thereafter from radioactive decay, allowing the date of death or fixation to be estimated. The initial C-14 level for the calculation can either be estimated, or else directly compared with known year-by-year data from tree-ring data (dendrochronology) to 10,000 years ago, or from cave deposits (speleothems), to about 45,000 years of age. A calculation or (more accurately) a direct comparison with tree ring or cave-deposit carbon-14 levels, gives the wood or animal sample age-from-formation. The technique has limitations within the modern industrial era, due to fossil fuel carbon (which has little carbon-14) being released into the atmosphere in large quantities, in the past several centuries. Carbon-14 and fossil fuelsMost man-made chemicals are made of fossil fuels, such as petroleum or coal, in which the carbon-14 has long since decayed. However, oil deposits often contain trace amounts of carbon-14 (varying significantly, but ranging from 1% the ratio found in living organisms to undetectable amounts, comparable to an apparent age of 40,000 years for oils with the highest levels of carbon-14).[citation needed] This may indicate possible contamination by small amounts of bacteria, underground sources of radiation (such as uranium decay), or other unknown secondary sources of carbon-14 production. Presence of carbon-14 in the isotopic signature of a sample of carbonaceous material therefore indicates its possible contamination by biogenic sources or the decay of radioactive material in related geologic strata. Carbon-14 and nuclear testsThe above-ground nuclear tests that occurred in several countries between 1955 and 1963 dramatically increased the amount of carbon-14 in the atmosphere and subsequently in the biosphere; after the tests ended the atmospheric concentration of the isotope began to decrease. This enabled the determination of the birth year of a deceased individual: the amount of carbon-14 in tooth enamel is measured with accelerator mass spectrometry and compared to records of past atmospheric carbon-14 concentrations. Since teeth are formed at a specific age and do not exchange carbon thereafter, this method allows age to be determined to within 1.6 years. This method only works for individuals born after 1943,[7][8] and it must be known whether the individual was born in the Northern or the Southern Hemisphere. Carbon-14 in the human bodySince essentially all sources of human food are derived from plants, the carbon that comprises our bodies contains carbon-14 at the same concentration as the atmosphere. The beta-decays from this internal radiocarbon contribute approx 1 mrem/year (.01 mSv /year) to each person's dose of ionizing radiation.[9] This is small compared to the doses from potassium-40 (0.39 mSv/year) and radon (which vary). Carbon-14 can be used as a radioactive tracer in medicine. In the urea breath test, a diagnostic test for Helicobacter pylori, urea labeled with approx 1 μCi (37kBq) carbon-14 is fed to a patient. In the event of a H. pylori infection, the bacterial urease enzyme breaks down the urea into ammonia and radioactively-labeled carbon dioxide, which can be detected by low-level counting of the patient's breath.[10] See alsoReferences
Categories: Isotopes of carbon | Environmental isotopes |
|||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Carbon-14". A list of authors is available in Wikipedia. | |||||||||||||||||||||||